Topical Application of Aprepitant Inhibits Erlotinib-induced Facial Dermatitis/Hair Loss
Iu Tong Mak*, Jay H. Kramer*, Joanna J. Chmielinska*, William B. Weglicki
Department of Biochemistry and Molecular Medicine, The George Washington University, School of Medicine and Health Sciences, Ross Hall, Rm 228, 2300 Eye Street, NW. Washington DC 20037; USA
Abstract
Erlotinib, an EGFR-TKI, has been used as an effective anti-tumorigenesis agent against several cancers including lung, colon, head and neck. However, it has been reported to cause significant and severe cutaneous side effects. Our previous studies implicated substance P, a neuropeptide, as a significant mediator of skin toxicity. Our present study was designed to determine if the topical application of aprepitant, a specific substance P receptor blocker, would be protective against these skin side effects. Erlotinib in the diet was administered to the rats for 12 weeks. Facial rash and hair loss began to occur after 6 weeks and were most severe at 12 weeks when animals were sacrificed. Topical treatment of aprepitant to the facial area 3 times a week showed dose-dependent and progressive inhibition up to 70% of the induced dermatitis/hair loss. These results were comparable to the effects produced by oral doses in our prior study. At sacrifice, we also found significant elevations of neutrophil superoxide, that were inhibited by topical aprepitant, along with elevated plasma 8-isoprostane levels, that were also suppressed. Facial skin samples revealed increased leukocyte (CD11b positive) infiltration in the erlotinib-treated rats, which were substantially reduced by the topical aprepitant. In conclusion, the indicators of reactive oxidative species (ROS) suggest that neurogenic inflammation played a critical role in causing EGFR-TKI-induced toxicity; it also confirmed that the systemic inhibition of ROS production due to blockade of substance P action was significantly protective against the dermatitis/hair loss pathology.
Introduction:
Erlotinib (Tarceva), a tyrosine kinase inhibitor (TKI) targeting the epidermal growth factor receptor 1 (EGFR1), is an orally available drug and has been used for treating several cancers including lung, colon, head and neck1. However, it has also been reported that erlotinib can induce severe cutaneous toxicity, manifested as acneiform skin rash, pruritis, and alopecia2,3, which in severe cases, might profoundly diminish patients’ quality of life to negatively impact adherence to the cancer therapy. In our recent published work4, we reported that erlotinib-mediated dermatitis/hair loss in a rat model was induced when erlotinib was administered in the diet. We also demonstrated that the dermatitis/hair loss was substantially suppressed by oral intake of aprepitant (Emend) along with significant inhibition of substance P (SP) receptor-mediated ROS activation. The present study was designed to determine, from a potential clinical treatment point of view, whether the induced dermatitis/hair loss could be mitigated by topical application of aprepitant. Thus, in this study, we applied aprepitant topically to the facial area, and assessed the degree to which such treatment could prevent the cutaneous and systemic side effects.
Materials and Methods
Methodology/Materials and Animal Treatment:
All animal studies were performed according to the principles in the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and received prior approval (IACUC # A2019-001 expired on 9/17/22) from The George Washington University Institutional Animal Care and Use Committee (GWU IACUC). Male Sprague Dawley rats were purchased from Hilltop Lab Animals, Inc. (Scottdale, PA) through the GWU Animal Research Facility (ARF). All rats used for the studies were normal healthy animals. Following quarantine, all age-matched rats (125-175 g) received an ad libitum Mg normal diet (25 mmol magnesium oxide/kg food considered 100% recommended daily allowance for rodents, TD 93104) obtained from Envigo-Teklad Laboratory (Madison, WI) containing extracted casein as the diet base and essential vitamins and nutrients 4. For the oral drug studies, this diet was supplemented with erlotinib (120 mg/kg diet, erlotinib obtained from Cayman Chem. MI) to reach a starting dose of 10 mg/kg/day. Purified aprepitant (Sigma) was used to prepare different concentrations (0.222 to 1 mg/ml) in specified topical solutions (described in Results). Rat groups included: controls (Ctl), erlotinib-treated alone (Erl), erlotinib + topical aprepitant-treatment at different doses (0.33 to 1 mg/kg BW/day), and erlotinib treated + topical vehicle solution alone (Erl + Veh). Rats were housed individually, and body weight and food consumption recorded daily to determine actual drug dosage: With a starting oral dose of 10 mg/kg/day of erlotinib, the range average dose estimate during 12-week exposure was 7- 5.7 mg/kg/day (reduced due to body weight gain). For the topical aprepitant studies, the low and high doses of aprepitant were applied to attenuate the erlotinib-induced cutaneous and systemic side effects. Topical application was initiated one week after starting oral erlotinib intake in the diet. When under anesthesia (2% isoflurane, EZ Anesthesia System plus nose cone, Palmer, PA), the aprepitant solution (in Solution C) was delivered through micropipette tips on the unshaven facial areas around the nose, in between and beneath eyes, forehead, and the upper back near the head and ears. The estimated area of application was about 4 - 5 cm2. Topical treatment was conducted 3 times a week (M-W-F). Each time a total volume of 105 µl solution [0.22% (low dose) to 0.67 % (high dose)] of aprepitant was applied. The weekly topical doses of aprepitant were estimated to be 7 mg/kg rat and 2.33 mg/kg (equivalent to 1 mg/kg/day and 0.333 mg/kg/day, respectively), for the high and low doses of aprepitant.
Measurement of Systemic oxidative indices: PMN superoxide and plasma 8-isoprostane
At the end of 12 weeks, blood samples (~ 8-10 ml in heparin plus aprotinin [10.74 units/ml and 0.016 units/ml, respectively] containing BD vacutainer SST tubes) were obtained by cardiac puncture from anaesthetized (by 2-5 % isoflurane) and heparinized rats (0.3-0.4 ml 358 units/ml heparin in 0.9% NaCl, i.p.). Samples were centrifuged at 3,500 rpm, 10 min, 4oC. to obtain plasma aliquots which were stored at -80oC for biochemical assays later. Neutrophils freshly isolated from whole blood samples were used for assessment of superoxide anion production.
Neutrophil basal superoxide generating activity: At sacrifice, neutrophils (from 3 ml of whole blood from each rat) were obtained by a step-gradient centrifugation for 30 min at RT 4-6. The neutrophil rich fractions were treated briefly (30 sec) with hypotonic solution (10% PBS) to remove the contaminating RBCs. Superoxide anion generating activity from the neutrophils (0.5-0.75 x 106/ml) was determined in a Na-phosphate buffer (pH 7.6) containing 5 mM glucose, 1 mM MgCl2, 1 mM CaCl2, and 75 cytochrome c ± 50 µg SOD. Superoxide generating activity was estimated as SOD-inhibitable reduction of cytochrome c using the extinction coefficient: E550=2.1x104M-1cm-1 5,6.
8-Isoprostane determination: Plasma samples were diluted 5- and 10-fold using the ELISA assay buffer (from Cayman Chemical). Free 8-isoprostane levels for the samples were determined using an 8-isoprostane ELISA Kit from Cayman Chemical, Ann Arbor, MI, (Item no 516351) and were calculated according to standard curves 4-6.
Facial/Head digital Images and Grading Skin/Hair Loss Changes:
During treatment at 8 and 12 weeks, the anesthetized rats from each group were placed ventral side down and a photographic image was obtained primarily of the facial/head region (at an approximate distance of 6 inches) of the animals 4. The animals were then placed in their holding cages until fully recovered. The camera feature of the iPhone 6s Plus was used with high dynamic range (HDR to blend the best of 3 separate exposures into a single picture). The rear camera has a 12 mega-pixel sensor, 1.22 µm pixels, an f/2.2 aperture, and includes optical image stabilization. Stored digital photographic images of all rats were evaluated by two blinded investigators and average scores for visual skin/hair loss changes were graded using the National Cancer Institute Common Toxicity Criteria (5, NCI, Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: November 27, 2017) 7,8. Severity of facial dermatitis 4 was scored on a 0 (none) to 4 (worse) scale based on macular or papular eruption or erythema and hair loss (alopecia) was similarly scored 0 (none) to 4 (worse, loss from >50% facial surface area). Dermatitis and hair loss values were averaged for each group.
Skin Sampling and CD11b Assessments:
Skin sample harvesting: Immediately following sacrifice, the facial area was shaved with an electric razor and treated with depilatory cream (< 2 min exposure), then cleaned/rinsed. The skin was removed with a scalpel from the facial area (from above the nose between the eyes and towards the forehead). The triangular-shaped tissue sample had an approximate area of 4.5 cm2. The skin samples were quickly rinsed in PBS, embedded in OCT compound, frozen by immersing in 2-methylbutane on dry ice and kept at -80°C until used. Cryosections, 5 µm thick, were stained immunohistochemically 4. Immunostaining for CD11b: Defrosted tissue sections were fixed in cold (-20°C) acetone and rinsed in TBS. The endogenous horseradish peroxidase activity was quenched by 5-minute incubation with 0.03% hydrogen peroxides in TBS and sections were rinsed in TBS. Then the non-specific binding was blocked with blocking buffer (10% horse serum, 1% BSA and 0.05% Triton 100X in TBS) for 30 minutes and the mouse anti rat CD11b (1:250 in TBS) antibody (Millipore, Tamecula, CA) was applied through overnight incubation at 4°C in the humidity chamber. After multiple washes in TBS, Vectastain Elite ABC HRP Kit followed by ImmPac DAB Substrate HRP Kit (Vector Laboratories Inc., Burlingame CA) were used to label and visualize the CD11b antigen (brown color). For counterstaining hematoxylin was used (blue nuclei). Stained slides were dehydrated in graded alcohols and 3 changes of xylenes, mounted with mounting medium and let dry.
Samples were examined under the Nikon Eclipse Ti microscope at 10x magnification scale bar = 100µm), and multiple images were taken with a digital camera (Nikon Digital Sight DS-U3). The areas (number of pixels) of positively stained parts of the skin sections were measured using ImageJ program (10 different microscopic fields/section for each rat, 5 rats/group). ImageJ short for image processing and analysis in Java, http://imagej.nih.gov (ImageJ) is a reliable program for computer-assisted analysis of microscopic function, allows one to manually filter and mark positively stained areas of interest in the tissue section. When satisfied with the color threshold, the calculation of number of pixels in the area of interest divided by number of pixels in entire tissue area and multiply by 100 provides the percent of the positively stained area. Results from analysis of ten fields in each micrograph from five animals per group were used for quantification. Changes in treatment groups compared to control (Ctl =100%) were assessed with respect to immunohistochemical staining for CD11b positive inflammatory cells.
Statistical Analyses
All data are the means ± SEM of 4-5 rats per group, and were checked by F test for equality of variation. Statistical differences were analyzed by two-tailed Student t-test. Values of p<0.05 were considered significant.
Results:
Pilot/study with transcutol/DMSO based solution of aprepitant and application topically on the face area to determine if protective against erlotinib-induced dermatitis and hair loss.
In an initial series of study, we attempted to determine whether purified aprepitant at 0.5% dissolved in a transcutol (70%) based solution (which also contained 10% DMSO and 19% acetone), applied on the facial area of the rats would be protective. Topical applications were conducted 2-3 times per week up to 12 weeks as described in Methods. Indeed, we found that these topical applications of aprepitant at an average dose of 0.7 mg/kg/day to be quite effective in providing inhibition of facial lesion development and hair losses by a semi-quantitative assessment (data not shown). It was estimated that the protective efficacy was about 70% attenuation and this was comparable to our prior study with oral aprepitant treatment at 2 mg/kg/day 4. Transcutol was employed due to its well-known properties: low toxicity and the ability to facilitate drug transfer into the dermal layer10. DMSO was used for its superior solubility for aprepitant; whereas acetone was included for its ability to facilitate evaporation of the solvent from the skin 11. However, since both DMSO and acetone are considered to be less desirable for clinical dermal application, a new formulation of the aprepitant vehicle solution was obtained through collaboration with Tergus Pharma. This new solution still used transcutol as one of the primary components, but other components were replaced by benzyl alcohol (2%) and propylene glycol 56-57%. Benzyl alcohol (a topical anesthetic with anti-parasitic properties) is FDA approved for clinical use up to 5%. Propylene glycol is also FDA approved and it has been used in preparations of cosmetics and even used as a food additive 10. The new solution (solution “C”) can dissolve aprepitant with an upper limit of 2% (w/v). For the main series of study, two different concentrations (0.22 % and 0.67% aprepitant solutions) were used to provide the selected low and high doses of aprepitant for topical application to the facial area 3 times per week.
Treatment with erlotinib and topical aprepitant on food consumption and body weight gain:
The effects of erlotinib with high or low doses of topical aprepitant on food intake and weight gain were described in Fig 1A and 1B. As shown in Fig 1-A. food consumption for rat groups receiving erlotinib alone ± vehicle C or plus high or low topical doses of aprepitant were not different among themselves; however, the average food intakes for all groups receiving erlotinib were slightly lower (~10% lower than the control and achieved significance at 8 weeks (Fig 1A). Also, body weight gains for erlotinib alone or ± aprepitant treatment were lower by 10-20 % towards 8 to 12 weeks (Fig 1B).
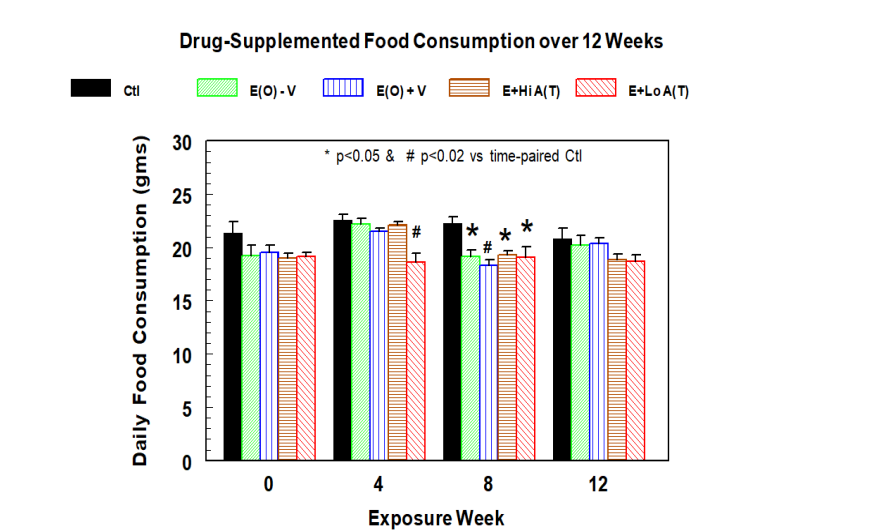
Figure 1(A): Effects of dietary treatment of erlotinib (10 mg/kg BW/day) with or without facial topical application of high dose (1 mg/kg BW/day) or low dose (0.33 mg/kg BW/day) of aprepitant dissolved in solution C on food consumption. Data are averages of 5 ± SEM; * p<0.05 vs Ctl, # p<0.02 vs time-paired Ctl.
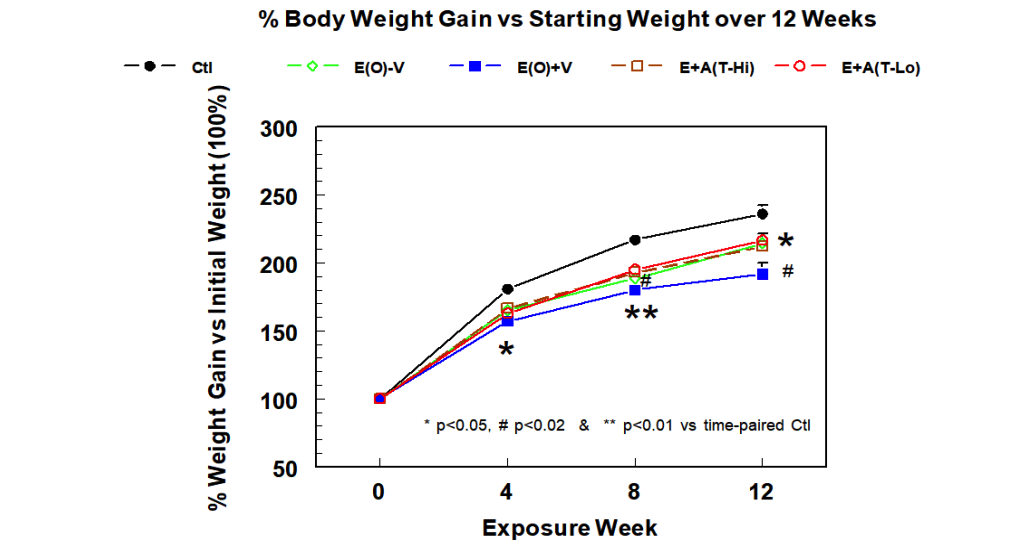
Figure 1(B): Effects of dietary treatment of erlotinib (10 mg/kg BW/day) with or without facial topical application of high dose (1 mg/kg BW/day) or low dose (0.33 mg/kg BW/day) of aprepitant dissolved in solution C on weight gain in rats up to 12 weeks. Data are averages of 5 ± SEM; * p<0.05 vs Ctl, # p<0.02 vs time-paired Ctl.
Since, aprepitant was applied locally within a relatively small area, we expected that the beneficial effects would be insufficient to reverse the entire systemic impact, including lowering body weight caused by erlotinib treatment. In the pilot study, we found that an intermediate dose of aprepitant (0.7 mg/kg/day) dissolved in DMSO/acetone/transcutol also did not prevent the weight loss (although providing substantial protective effects on the induced lesion/hair loss). However, we did notice a further slight weight loss in the E(O)+ V vs the E(O) -V group. We interpret that this effect might be due to the additional anesthesia/physical stress caused by the topical application procedure. Nevertheless, there was no significant difference (n=4) in body weights at 12 weeks between Erl(O) - Veh (436g ± 13.76) and Erl(O) + Veh (394g ± 16.39, 9.6% lower).
Effects on oxidative indices: Neutrophil activity and plasma 8-isoprostane levels.
Similar to what was observed in our previous study 4, neutrophils isolated from the erlotinib-treated rats at 12 weeks displayed a 3.53-fold higher basal superoxide generating activity (Fig 2A). Interestingly, the activity for the neutrophils of the Erl plus topical veh C was found to be slightly higher (3.86-fold higher); this was not statistically different from the Erl alone. We found that topical treatment of the erlotinib rats with the high dose of aprepitant (1 mg/kg/day) lowered the neutrophil activity to 1.14-fold of controls, which was >70% suppression (p<0.01) of the basal activity of superoxide generation compared to the Erl+ veh “C” group. Most interestingly, at the low dose of 0.33 mg/kg/day, aprepitant applied topically still provided a 24% (p<0.02) reduction of the superoxide activity (Fig 2A). Consistent with the changes in the basal neutrophil activities, we found that plasma 8-isoprostane levels of the erlotinib treated rats without and with the veh C, were elevated 3.3-fold and 3.4-fold, respectively, compared to controls (Fig 2 B). The high dose of aprepitant lowered the Erl-mediated increase by 60% (p<0.01), whereas the lower dose of topical aprepitant also lowered the plasma isoprostane levels by 26% (p=0.051).
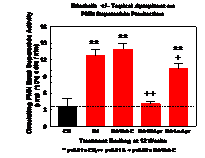
Figure 2(A): Changes in basal neutrophil superoxide generation activity caused by erlotinib with or without topical treatment of high or low doses of aprepitant after 12 weeks of exposure in rats. Other conditions were described in Materials and Methods. Values are means of 5 ± SEM; **p<0.01 vs Ctl, +p<0.05, ++p<0.01 vs Erl+ veh C.
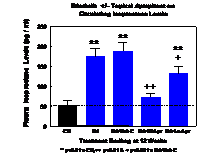
Figure 2(B): Changes in circulating 8-isoprostane levels caused by erlotinib with or without topical treatment of high or low doses of aprepitant after 12 weeks of exposure in rats. Other conditions were described in Materials and Methods. Values are means of 5 ± SEM; **p<0.01 vs Ctl, +p<0.05, ++p<0.01 vs Erl+ veh C.
Dose dependent protective effect of aprepitant in solution “C” against erlotinib-induced dermatitis/hair loss.
In this rodent model, we have demonstrated that the dermatological toxicity of erlotinib could be generated in rats, especially on the facial area4. We believe hair loss is associated with facial dermatitis. The skin underlying areas of thinning or absence of hair, for the most part, appears thickened, reddish and inflamed.
In the present study, with weekly observation of the development and progression of facial dermatitis and hair loss in the erlotinib-treated rats, we studied the effects of the two doses of topical aprepitant on the facial area.
The dermatological changes were noticed by 6 weeks in all erlotinib treated rats and the skin changes progressed in severity up to 12 weeks. The changes included rough coat, skin reddening /thickening, scabbing and crusting around nose and patchy alopecia (hair loss). At the end of 12 weeks, digital facial images for 4 animals per group were selected and represented in Fig 3. Scoring averages (± SEM) for both 8 weeks and 12 weeks animals were summarized by Table 1. The controls remained 0 lesion and 0 hair loss throughout the 12 weeks of experimental period. The results indicated that erlotinib alone resulted in significant increases in lesion scores averaging 1.98 at week 8 and progressed further to 2.9 at week 12. Interestingly erlotinib plus the vehicle solution C induced a slightly higher extent of dermatitis and hair loss with scores of 2.3 and 2.43 respectively at week 8 and both progressed to 3.1 at week 12 (Table 1). Most importantly, we found that topical treatment with the high dose of aprepitant reduced the lesion score to 0.73 at week 8 and to 0.725 at week 12, which were 69% and 75% (p< 0.01) decreases compared to the scores for Erl + solution C alone. At the low dose of aprepitant, modest reductions of lesions were also archived (33% to 40%) for weeks 8 and 12, but marginal significance was achieved only for week 12 (p=0.054). Rats receiving Erl alone resulted in a hair loss score of 2.24 at week 8 which progressed to 3.1 at week 12. With the addition of Veh C, the scores were 2.43 and 3.1 respectively, the high dose of aprepitant reduced the scores to 0.688 (week 8) and 0.75 (week 12) representing significant inhibition of 70% and 76% (p<0.01), respectively. Similar to its effects on the lesion development, the low dose aprepitant provided partial inhibition of hair loss at both week 8 (48%) and week12 (33%); marginal significance (p=0.054) was revealed only for the week 12 scores (Table 1).
Table 1: Effects of erlotinib (Erl) ± topical (T) aprepitant in vehicle C on facial skin dermatitis and hair loss in rats after 8 and 12 weeks of treatment.
|
Rat Groups (n= 4) |
8 Weeks |
12 Weeks |
||
|
|
Facial Skin Lesion |
Facial Hair Loss |
Facial Skin Lesion |
Facial Hair Loss |
|
Ctl |
0.00±0.00 |
0.00±0.00 |
0.00±0.00 |
0.00±0.00 |
|
Erl alone |
1.98±0.27** |
2.24±0.25** |
2.90±0.23** |
2.65±0.30** |
|
Erl+ Veh C |
2.30±0.44** |
2.43±0.48** |
3.10±0.25** |
3.31±0.20** |
|
Erl + Hi Apre (T) |
0.73±0.10**,+ |
0.69±0.08**,+ |
0.73±0.08**,++ |
0.75±0.15*,++ |
|
Erl + Lo Apre (T) |
1.38±0.08** |
1.30±0.09** |
2.00±0.44*,# |
2.08±0.43*,# |
|
Ctl= untreated control; Erl±Veh C=erlotinib (10 mg/kg BW/day in diet) with or without topical vehicle C; Erl+Hi Apre (T)=erlotinib + high dose of topical aprepitant (1 mg/kg BW/day) in vehicle C; Erl+Lo Apre (T)= erlotinib+low dose of topical aprepitant (0.33 mg/kg BW/day) in vehicle C. *P<0.05, **<0.01 vs Ctl; + p<0.05, ++<0.01 vs Erl + Veh C; # p<0.055 vs Erl + Veh C.
|
||||
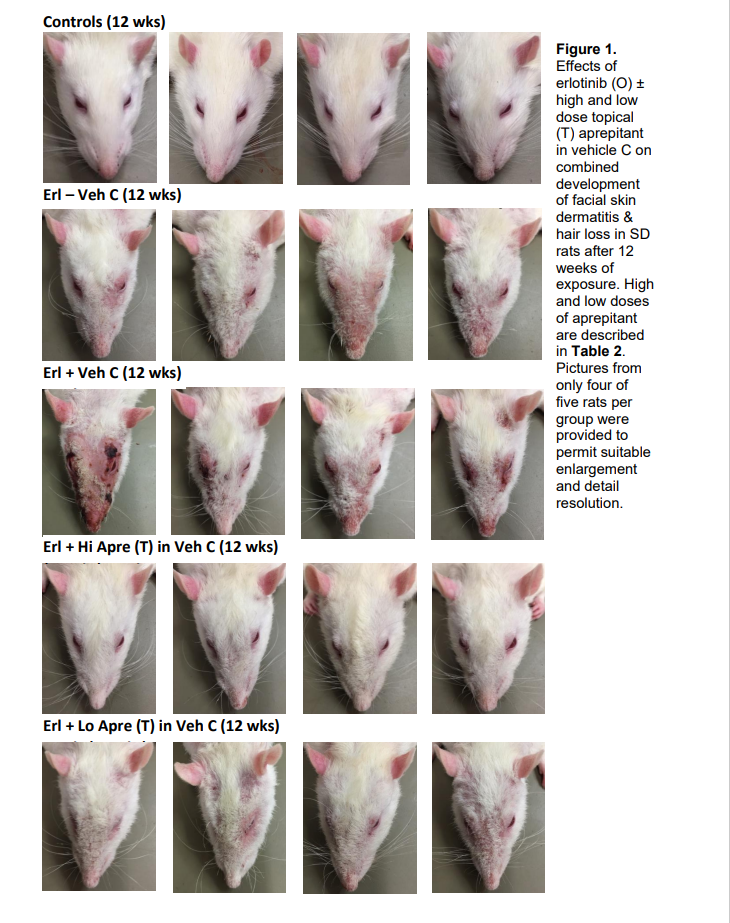
Figure 3: Digital images of rats indicating varying degrees of facial dermatitis and hair loss after 12 weeks of oral erlotinib ± high or low dose of facial topical application of aprepitant; 4 rats from each group were presented here.
Effect of topical aprepitant on CD11b positive cells in the facial skin of rats treated with erlotinib
Changes in the treatment groups compared to control (Ctl =100%) were assessed with respect to the level of immunohistochemical staining for CD11b positive inflammatory cells. The individual cells and small clusters were observed scattered within the epidermis and dermis. Number of pixels in the brown stained areas was normalized according to the total number of pixels in the entire tissue in the microscopic field and reported as percent of positive staining in the field. Representative micrographs of immunochemical staining for CD11b as a marker for inflammatory WBC infiltration in the facial skin samples from different groups of rats are displayed in Figure 4A. The results demonstrate differential changes in staining for CD11b positive cells in facial skin produced by topically applied aprepitant at two different doses in rats treated with oral erlotinib compared to those treated only with erlotinib or erlotinib + vehicle C. The quantitative analysis of observed changes is represented by Figure 4B.
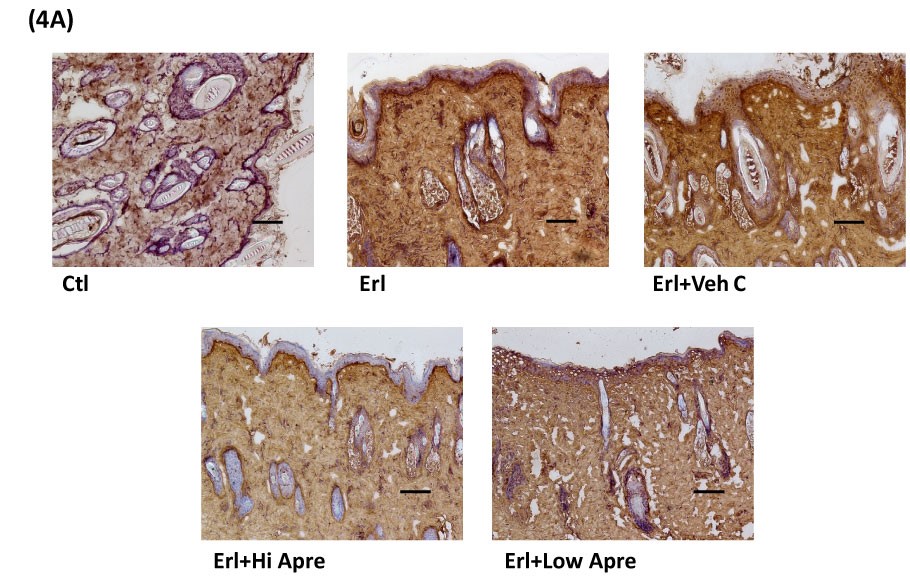
Figure 4(A): Representative micrographs of immunochemical staining for CD11b as a marker for inflammatory WBC infiltration in the facial skin from rats on erlotinib treated diet with two different topical doses of aprepitant (1mg/kg BW/day and 0.33mg/kg BW/day). CD11b (+) staining significantly increased in Erl and Erl+Veh C treated groups but was diminished in the topically applied aprepitant treatment. Scale 100 µm
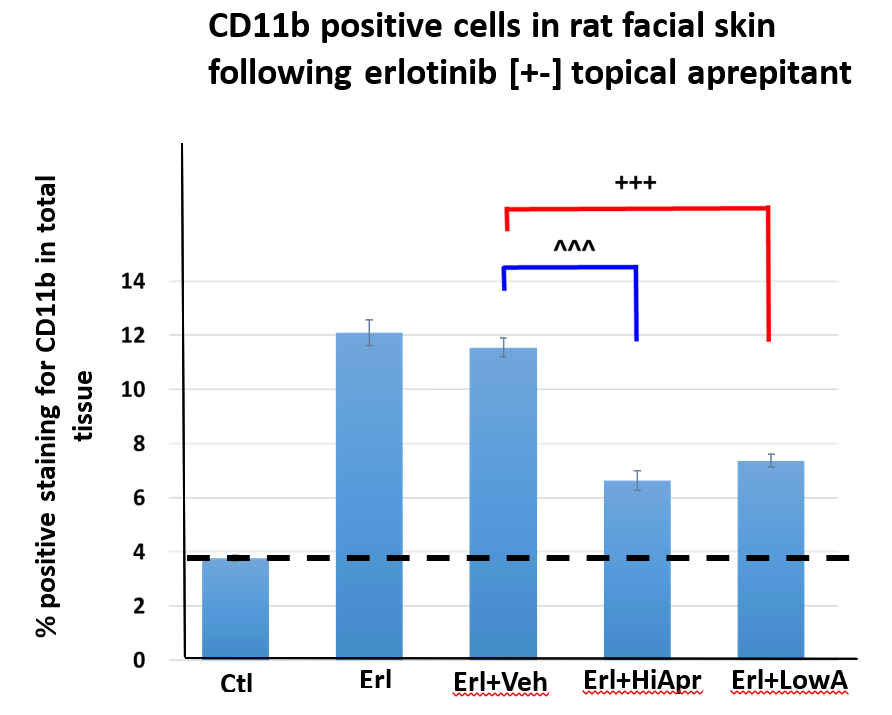
Figure 4(B): Quantitative analysis of the observed changes in CD11b positivity in the facial skin from rats on erlotinib treated diet with two different topical doses of aprepitant. ImageJ program was used to assess the areas (number of pixels) stained positively for CD11b (brown; 10 different microscopic fields/section for 5 rats/group).
Erlotinib alone and erlotinib + vehicle C produced highly significant increases of 3.21 -fold and 3.06- fold, respectively (p<0.005), in CD11b staining in the facial skin in comparison with the control group. Based on analysis of the positively stained areas, there was no significant difference (p=0.37) of CD11b staining in the skin samples from erlotinib alone and erlotinib + vehicle C treated animals. Topical treatment with the high dose of aprepitant significantly (p<0.01) suppressed the staining by 43% compared with Erl+Veh C group; interestingly, even with the low dose of aprepitant, the extent of the CD11b staining was reduced by 36% but is statistically not different (p=0.1) from the effect produced by the high dose of aprepitant. In data not shown, we also found that aprepitant, dissolved in the DMSO/acetone/transcutol, at an intermediate dose (0.7 mg/kg/day), substantially suppressed the intensity of CD11b expression by 56% compared to the erlotinib treated group.
Discussion
Erlotinib has been used mostly as an important therapeutic agent for lung cancer to achieve a significant overall survival benefit 1,12. Although generally well tolerated, erlotinib, and other EGFR inhibitors, were frequently associated with the development of cutaneous side effects. These changes were often regarded as surrogate markers for clinical outcome 12. To deal with the induced cutaneous toxicity, the available treatments with topical steroids and emollients achieved limited success 13,14. Our studies suggest that topical application of aprepitant, may inhibit the induced cutaneous toxicities of lesion development and hair loss. On a per body weight basis, the high dose of aprepitant used in this study appears to be on par with the clinical dose of aprepitant in Emend used for anti-vomiting therapy. However, it is well -known that rodents have at least 6-fold higher drug metabolizing activity 18. Therefore, we speculate that for clinical topical aprepitant application, the effective dose required would be much lower. We believe that the primary effect of topical aprepitant application can suppress the dermal expression of neurogenic inflammation. The dermis contains C fiber nerve endings which are widely distributed 14-17. In non-cancer patients with similar skin pathology, SP-related inflammation was reported 15: “The skin of atopic dermatitis lesion is hyper innervated with increased SP and CGRP-positive nerve fibers in the epidermis and papillary dermis …” with elevated plasma SP. In our previous study, we showed that circulating SP was upregulated by erlotinib treatment4. In a separate study 19 , we found that SP-receptor expression on the activated neutrophils, which was CD11b positive, was also enhanced. These combined results led us to conclude that activation of the neutrophil was promoted by the neurogenic peptide SP. In addition, we propose that erlotinib’s EGFR1 inhibition of the renal TRPM6 EGF-dependent resorption may permit wasting of magnesium20 that may activate the neuronal release of substance P and CGRP into the local areas of the dermis. In the interstitial area the capillary system permits substance P to enter the circulation to stimulate the circulating leukocytes to generate superoxides and increase systemic cellular oxidative stress resulting in elevated 8-isoprostane from other injured cells. We did not have quantitative data to estimate the systemic absorption of aprepitant following topical application. However, the absorption of aprepitant must be substantial enough to be able to partially prevent the WBC infiltration, and perhaps more importantly afford inhibition of local ROS-generation which promoted injury of epidermis and hair follicles. In addition, the absorption of lipophilic aprepitant into the capillary circulation enables blockade of the substance P-mediated leukocyte production of superoxide oxidative injury, which results in lower 8-isoprostane plasma levels. These data, showing systemic prevention of substance P-mediated oxidative stress may be protective, not only of circulating blood cells but other organs that may receive modest exposure to doses of circulating aprepitant.
CD11b is considered a marker of myeloid-lineage cells21, including neutrophils, WBC, monocytes/macrophages, along with NK cells and a subset of CD8+ T cells. We used enhanced CD11b histological positivity compared to controls as an indicator for WBC/monocytes infiltration, that was likely due to facilitated infiltration of neutrophils, monocytes/macrophages and T cells14,17. The histopathology studies (Fig 4) show that both high and low doses of topical aprepitant are able to significantly inhibit the increased number of CD11b positive cells in erlotinib-treated animals. This suggested that aprepitant easily crosses the local skin barrier even at a lower dose likely due to the highly lipophilic property of aprepitant. This also suggests that the topical delivery of this anti-inflammatory drug may be employed in other clinical applications that require antioxidative treatment for systemic disorders of excessive free radical stress from neurogenic inflammation. The vehicle C used to deliver aprepitant topically did not have any significant effect by itself on the CD11b cell levels in the skin of erlotinib -treated animals. Overall, our data also indicated that substance P alone played a significant role in facilitating the infiltration of the CD11b positive WBCs/monocytes into the facial dermis and which participated in the development of skin lesions and hair loss. Interestingly, with the low dose of topical aprepitant, the suppression of CD11b expression was almost as efficient as the high dose of aprepitant, yet the extent of dermatitis was only modestly protected, this suggests that the extent of anti-radical effects produced might also play an important role to prevent the induced dermatitis/hair loss.
In conclusion, we have presented substantial evidence that topical application of aprepitant may serve as an effective intervention against EGFR-TKI-induced cutaneous toxicity. We submit that the findings are novel since aprepitant is applied on the skin surface rather than given orally; it is perceived that the potential risk of interference with the anti-tumor activity of the oral EGFR-TKI agents would be diminished. Overall, the potential for new clinical studies to confirm the benefits of topical application of aprepitant is most promising to mitigate the EGFR-TKI-induced cutaneious toxicities and maximize the benefits of EGFR-TKI anti-cancer therapy.
Acknowledgement: Funded by Hoth Therapeutics, this study is patent protected and formation/administration methods have also been covered and filed by Hoth Therapeutics.
Author contribution: All authors contributed to conception and design of research. ITM, JHK, JJCh: performed experiments, collected and analyzed data. ITM, JHK, JJCh, WBW: interpreted results. ITM: drafted initial version of manuscript. ITM, JHK, JJCh, WBW: edited, revised and approved final version of manuscript.
Conflict of Interest: WBW: Consultant for Hoth Therapeutics Inc. USA. The sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, and in writing of the manuscript.
References
- Schettino C, et al, Erlotinib: An EGF receptor tyrosine kinase inhibitor in non-small-cell lung cancer treatment. Rev. Respir. Med, 2: 167-178 (2008).
- Kiyohara Y, et al. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. A. Acad. Dermatol. 69: 463-472 (2013).
- Jacouture M, Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting skin, oral mucosa, hair and nails. J. Clinical Dermatology 19 (Suppl 1): 531-539 (2018).
- Chmielinska JJ, et al. Substance P receptor blocker, aprepitant, inhibited cutaneous and other neurogenic inflammatory side effects of the EGFR1-TKI, erlotinib. Mol Cell Biochem 465: 175-185 (2020).
- Mak IT, et al. Mg-supplementation Attenuates Ritonavir-induced Hyperlipidemia, Oxidative Stress and Cardiac Dysfunction in Rats. J. Physiol. Regul. Integr. Comp. Physiol. 305: R1102–R1111 (2013).
- Weglicki WB, et al. The EGFR tyrosine kinase inhibitor tyrphostin AG-1478 causes hypomagnesemia and cardiac dysfunction. J. Physiol. Pharmacol. 90: 1145-1149 (2012).
- Pérez-Soler R, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. 10(5): 345-356 (2005).
- Brown AP, et al., Cutaneous Lesions in the Rat Following Administration of an Irreversible Inhibitor of ErbB Receptors, Including the Epidermal Growth Factor Receptor. Toxicologic Pathology 36: 410-419 (2008).
- Mak IT, et al. AZT-Induced cardiovascular toxicity - attenuation by Mg-supplementation. Cardiovascular Toxicol. 9: 78-85 (2009).
- Zhang Y, et al. N Transcutol® P/Cremophor® EL/Ethyl Oleate-Formulated Microemulsion Loaded into Hyaluronic Acid-Based Hydrogel for Improved Transdermal Delivery and Biosafety of Ibuprofen. AAPS Pharm Sci Tech. 21(1)1584-8 (2019).
- Acetone - Wikipedia https://en.wikipedia.org/wiki/Acetone; Dimethyl sulfoxide - Wikipedia https://wikipedia.org/wiki/Dimethyl_sulfoxide; Benzyl alcohol - Wikipedia https://en.wikipedia.org/wiki/Benzyl_alcohol.
- Macdonald JB, et al. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 72(2): 203-18 (2015).
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 69(3): 463-72 (2013).
- Marzano AV, et al. Mechanisms of Inflammation in Neutrophil-Mediated Skin Diseases. Front Immunol 10: 1059. (2019).
- Choi JE, Di Nardo AD. Skin neurogenic inflammation, Seminars in Immunopathology 40: 249-259 (2018).
- Pojawa-GoÅab M, Jaworecka K, Reich A. NK-1 Receptor Antagonists and Pruritus: Review of Current Literature. Ther. (Heidelb) 9:391–405 (2019).
- Gupta K, Harvima Mast cell-neural interactions contribute to pain and itch. Immunol Rev. 282(1):168-187(2018).
- Food and Drug Administration. U.S. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER) Table 1: Conversion of Animal Doses to Human Equivalent Doses Based on Body Surface Area: In: Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 1–27, (2005). http://www.fda.gov/cder/guidance/index.htm.
- Weglicki WB,et al. The role of magnesium deficiency in cardiovascular and intestinal inflammation. . Magnes Res.23(4): S199-206, (2010).
- Henrik D, et al. Effects of the EGFR Inhibitor Erlotinib on Magnesium Handling J Am Soc Nephrol. 21(8): 1309-16. (2010).
- Duan M, et al. CD11b immunophenotyping identifies inflammatory profiles in the mouse and human lungs Mucosal Immunology 9:550–563 (2016).
