The Dawning of a New Enterprise: RNA Therapeutics for the Skin
Rachel E. Kieser1,2,5#, Shaheerah Khan1,2,5#, Nada Bejar1,2,5#, Daniel L. Kiss1,2,3,4,5*
1Center for RNA Therapeutics,
2Department of Cardiovascular Sciences,
3Weill Cornell Medical College, New York, NY, USA
4Houston Methodist Cancer Center, Houston, TX, USA
5Houston Methodist Academic Institute, Houston Methodist Research Institute, 6670 Bertner Ave, R10‑113, Houston 77030, TX, USA
#These authors have contributed equally
Abstract
Despite being under development for decades, RNA therapeutics have only recently emerged as viable drug platforms. The COVID-19 mRNA vaccines have demonstrated the promise and power of the platform technology. In response, novel RNA drugs are entering clinical trials at an accelerating rate. As the skin is the largest and most accessible organ, it has always been a preferred target for drug discovery. This holds true for RNA therapies as well, and multiple candidate RNA-based drugs are currently in development for an array of skin conditions. In this mini review, we catalog the RNA therapies currently in clinical trials for different dermatological diseases. We summarize the main types of RNA-related drugs and use examples of drugs currently in development to illustrate their key mechanism of action.
Introduction
The discovery and development of novel RNA therapy candidates has accelerated since the success of the COVID-19 mRNA vaccines 1. RNA therapeutics have been tapped to provide novel approaches to treat various dermatological conditions where conventional treatments have offered little success. For example, diabetic patients suffer from poor wound healing, which correlates with an increased chance of infections leading to amputation. In addition, metastatic melanoma is one of the deadliest forms of skin cancer with a 5-year survival rate of less than 5% 2. It is inherently resistant to radiotherapy and chemotherapy with median survival reduced to approximately 10 months 2. Novel therapies are vital to treat these patients, with several candidate RNA therapies showing promising results.
The rapid advancement of RNA-based therapies underlines their inherent advantages. For example, (1) they have a transient effect, (2) present no risk of insertional mutagenesis, (3) are easy to develop and manufacture, and (4) are cost-effective. These traits make them attractive options for development. Here we summarize the RNA-based therapies currently in clinical trials for skin conditions (Table 1), described are the main categories of RNA therapeutics: RNAi (siRNA and miRNA), antisense oligonucleotides (ASO), messenger RNA (mRNA), and provide examples detailing their mechanisms of action (Figure 1).
Table 1: RNA-based therapies currently in clinical trials for skin conditions
|
Drug name |
Trial Phase |
Clinical trial # |
Status (Jan, 2023) |
Skin Condition |
Company |
Mechanism of action |
Alternative names of drug [38, 39] |
|
|
RNA interference (RNAi) |
||||||||
|
LEM S401 |
Phase I |
NCT04707131 |
Active |
Hypertrophic Scar & Keloid |
Lemonex |
Encapsulated siRNA targeting CTGF to reduce fibrosis |
LEM-S401; siRNA encapsulated in DegradaBALL |
|
|
PH 762 |
Phase I |
CSET 3432 |
Active |
Malignant melanoma & Squamous cell cancer |
Phio Pharmaceuticals |
Self-delivering siRNA reducing the expression of PD-1 to enhance T-cells anti-tumor activity (also tested as an Adoptive Cell Therapy) |
PH 762 adoptive cell therapy; PH-762; PH762-ACT; RXI 762-ACT |
|
|
TD101 |
Phase I |
NCT00716014 |
Completed |
Pachyonychia Congenita |
Transderm/International Pachyonychia |
siRNA targeting one disease-causing mutation of keratin 6a (K6a), the N171K mutant |
Pachyonychia congenita siRNA; Reveker; sdTD-K6a.513a.12; siRNA sdTD101; TD-K6a.513a.12; TD101 |
|
|
MRX 34 |
Phase I/II |
NCT02862145/NCT01829971 |
Terminated |
Melanoma |
Mirna therapeutics (Synlogic) |
Encapsulated synthetic miR-34a mimic- Tumour suppressor gene modulators |
miR-34 - Synlogic; miR-34 mimic - Synlogic; MRX-01; MRX34 |
|
|
BMT101 or OLX101A |
Phase II |
NCT04012099/NCT04877756 |
Active |
Hypertrophic scars |
Olix/Hugel |
Cell penetrating asymmetric siRNA targeting human CTGF to reduce fiborsis |
asiRNA therapeutics - OliX Pharmaceuticals; BMT 101; cp-asiRNA therapeutics - OliX Pharmaceuticals; OLX 10020; OLX 103; OLX 201; OLX 301; OLX 401; OLX 701;OLX10010; OLX101 |
|
|
STP 705 |
Phase II |
NCT04844840/NCT05196373/NCT04844983/NCT05421013 |
Active |
Squamous Cell Carcinoma, Keloids & hypertrophic scar, Fat sculpting |
Sirnaomics |
Two encapsulated siRNAs targeting TGF-β1 and Cox-2 to reduce fibrogenic response |
Anti-fibrosis RNA interference therapeutic - Sirnaomic; Cotsiranib; STP-705; STP-705LU; STP-705LV; STP705L |
|
|
Remlarsen |
Phase II |
NCT03601052 |
Completed |
Pathological fibrosis, Fibrous scar & Keloid |
miRagen Therapeutics (Viridian) |
miR-29b mimic to reduce collagen and other scarring proteins |
miR-29 replacement; MRG-201 |
|
|
RXI-109 |
Phase II |
NCT02030275/NCT02246465/NCT02079168 |
Completed/unknown |
Hypertrophic Scars & Keloids |
RXi Pharmaceuticals (Phio Pharmaceuticals) |
Self-delivering siRNA targeting CTGF to reduce fibrosis |
RXI 109; PH-109 |
|
|
Antisense Oligonucleotides (ASOs) |
||||||||
|
AST-005 |
Phase I |
NCT01290692 |
Completed |
Psoriasis |
Purdue Pharma,Exicure |
Nanoparticle-based spherical nucleic acid (SNA) to knockdown a tumor necrosis factor gene; Tumour necrosis factor inhibitors |
AST-005 |
|
|
MRG 110 |
Phase I |
NCT03603431 |
Completed |
wound healing |
Viridian Therapeutics; miRagen Therapeutics, Inc. |
Inhibitor of microRNA-92 |
Anti Mir92a; MRG-110; S95010 |
|
|
XCUR17 |
Phase I |
Unknown |
Completed |
Alopecia; Netherton syndrome; Psoriasis |
Dermelix Biotherapeutics; Exicure |
SNA targeted to mRNA encoding interleukin 17 receptor alpha (IL-17RA) |
XCUR 17 |
|
|
QR 313 |
Phase I/II |
NCT05529134 |
Active |
Epidermolysis bullosa |
Phoenicis Therapeutics; ProQR Therapeutics |
Designed to exclude exon 73 from the mRNA (exon skipping) and produce a functional C7 protein, thereby restoring the functionality of the anchoring fibrils |
PTW-002; QR-313; QRX-313 |
|
|
QR 313 |
Phase I/II |
NCT03605069 |
Terminated |
Fibrous scar & Keloid |
Phoenicis Therapeutics |
Designed to exclude exon 73 from the mRNA (exon skipping) and produce a functional C7 protein, thereby restoring the functionality of the anchoring fibrils |
PTW-002; QR-313; QRX-313 |
|
|
SB011 |
Phase II |
NCT02079688 |
Completed |
Atopic dermatitis |
Sterna Biologicals GmbH & Co. KG |
DNAzyme hgd40 targeting GATA3, a highly mutated transcription factor |
hgd40; SB-010; SB-011; SB-012 |
|
|
Cobomarsen |
Phase II |
NCT03837457 |
Terminated |
Cutaneous T-Cell Lymphoma; Dystropic Epidemolysis Bulosa |
Viridian Therapeutics |
Inhibitor of miR-155 |
MRG-106 |
|
|
PF-06473871 |
Phase II |
NCT02205476 |
Terminated |
Hypertrophic scar |
Pfizer |
Anti-CTGF antisense oligonucleotide |
EXC-001; PF-06473871; PF-6473871 |
|
|
Trabedersen |
Phase II/III |
NCT00844064 |
Completed |
Melanoma |
Oncotelic Therapeutics |
mRNA of the human TGF-β2 gene |
AP 2/09-DS; AP-12009; AP-2/09; OT-101; OT-201; Personalised dosing TGF-beta antisense; TGF-beta antisense |
|
|
Donidalorsen |
Phase III |
NCT05392114 |
Active |
Hereditary angioedema |
Ionis Pharma |
Reduce the production of prekallikrein |
Donidalorsen sodium; IONIS-PKK-L Rx; ISIS 721744 |
|
|
Tilsotolimod |
Phase III |
NCT04126876 |
Active |
Malignant melanoma; Anti-programmed cell refractory death protein 1 (PD-1) metastatic melanoma |
Idera/Bristol Myers Squibb |
Immunologic cytotoxicity; Toll-like receptor 9 agonists |
IMO-2125; IMO-2125 sodium; Tilsotolimod sodium |
|
|
Messenger RNA (mRNA) |
||||||||
|
Lipo-MERIT |
Phase I |
NCT02410733 |
Active |
Advanced Melanoma |
BioNTech SE |
mRNA encoding TAAs: NY-ESO-1, MAGE-A3, tyrosinase, and TPTE |
None |
|
|
BNT131 |
Phase I |
NCT03871348 |
Active |
Malignant melanoma |
BioNTech/Sanofi |
mRNA encoding cytokines: IL-12sc, IL-15sushi, IFNa and GM-CSF; BioNTech FixVac platform |
BNT-131; SAR-441000 |
|
|
mRNA-2752 |
Phase I |
NCT03739931 |
Active |
Immune Checkpoint Refractory Melanoma, Relapsed/Refractory Solid Tumor Malignancies |
Moderna Therapeutics (Collaborator: AstraZeneca) |
mRNA encoding OX40L T cell co-stimulator, IL-23, and IL-36γ pro-inflammatory cytokines; immunostimulant |
iTu triple combination - Moderna Therapeutics; mRNA intratumoral immuno-oncology therapeutics - Moderna Therapeutics; mRNA 2752; OX40L/IL-23/IL-36γ |
|
|
JCXH-211 |
Phase I |
NCT05539157 |
Active |
Malignant melanoma |
Immorna Biotherapeutics, Inc. |
self-replicating mRNA encoding cytokine IL-12 |
JCXH 211 |
|
|
RBL001/RBL002 |
Phase I |
NCT01684241 |
Completed |
Melanoma |
BioNTech RNA Pharmaceuticals GmbH (BioNTech SE) |
mRNA encoding TAAs: RBL001/RBL002 |
MERIT; RB0001; RB_0001; RBL-001/RBL-002; RBL-002/RBL-001 |
|
|
BNT121 |
Phase I |
NCT02035956 |
Completed |
Malignant Melanoma, Unresectable Malignant Melanoma stage IIIA-C and IV |
BioNTech RNA Pharmaceuticals GmbH (BioNTech SE) |
Poly-neo-epitopic encoding mRNAs specific to patient mutanome with and without the RBL001/RBL002 antigen-encoded mRNA |
BNT-121; IVAC MUTANOME; Melanoma RNA vaccine personalised - BioNTech/Ribological/TRON; Personalised melanoma vaccine - BioNTech/TRON |
|
|
NEO-PV-01 |
Phase I |
NCT02897765 |
Completed |
Malignant Melanoma |
BioNTech US Inc. (Collaborator: Bristol-Myers Squibb) |
mRNA encoding neoantigens for a personalized cancer vaccine to patient mutanome; immunoglobulin stimulant |
Neo Vax; NEO-PV-01; Neoantigen vaccine; Neoantigen-based vaccine - BioNTech; NeoAntigen-peptides; Personalized-neoantigen-cancer-vaccine-BioNTech; Personalized-neoantigen-vaccine-BioNTech |
|
|
NEO-PV-01 |
Phase I |
NCT03597282 |
Terminated |
Metastatic Melanoma |
BioNTech US Inc. (Collaborator: Apexigen, Inc.) |
mRNA encoding neoantigens for a personalized cancer vaccine to patient mutanome; immunoglobulin stimulant |
None |
|
|
Poly-ICLC |
Phase I |
NCT01970358 |
Completed |
Melanoma |
Patrick Ott, Md, Dana-Farber Cancer Institute; Oncovir, Inc. |
mRNA encoding neoantigens (up to 20 TAAs) for a personalized cancer vaccine to patient mutanome |
NeoVax; Hiltonol |
|
|
ECI-006 |
Phase I |
NCT03394937 |
Terminated |
Melanoma |
eTheRNA immunotherapies |
mRNA encoding TAAs: tyrosinase, GP100, MAGE-A3, MAGE-C2, and PRAME. Administered with mRNA encoding immunostimulants (TriMix) |
ECI 006 |
|
|
BNT122 |
Phase I/II |
NCT03815058/NCT03289962 |
Active |
Untreated Advanced Melanoma |
Genentech, Inc. (Collaborator: BioNTech SE) |
mRNA encoding neoantigens for a personalized cancer vaccine to patient mutanome; BioNTech iNest platform |
BNT-122; BNT122/RO7198457; Autogene cevumeran; Individualized Neoantigen Specific immunotherapy; iNeST; IVAC_M_uID; PCV RO7198457; Personalized cancer vaccine RO7198457; RG-6180; RG6180-1; RO-7198457 |
|
|
GM-CSF |
Phase I/II |
NCT00204516 |
Completed |
Malignant Melanoma |
University Hospital Tuebingen (Collaborator: German Research Foundation) |
mRNA encoding TAAs: melan-A, MAGE-A1, MAGE-A3, survivin, GP100, and tyrosinase |
Molgramostim; CSF 39300; GMC 89107; Leucomax; SCH 39300 |
|
|
National Cancer Institute (NCI)-4650 |
Phase I/II |
NCT03480152 |
Terminated |
Melanoma |
National Cancer Institute (NCI); Moderna Therapeutics |
mRNA encoding neoantigens (up to 15 TAAs) for a personalized cancer vaccine against those expressed by autologous tumor cells |
mRNA 4650; NCI 4650; mRNA-based PCV NCI-4650; NC-I4650 |
|
|
BNT111 |
Phase II |
NCT04526899 |
Active |
Melanoma Stage III, Melanoma Stage IV, Unresectable Melanoma, Anti-PD-1-refractory/ relapsed melanoma, malignant melanoma |
BioNTech SE (Collaborator: Regeneron Pharmaceuticals) |
mRNA encoding TAAs: NY-ESO-1, MAGE-A3, tyrpsinase, TPTE; BioNTech FixVac platform |
BNT-111; Lipo-MERIT; RB 0003; RB_0003; RBL 001/RBL 002/RBL 003/RBL 004; RBL001.1/RBL002.2/RBL003.1/RBL004.1; RNA-LPX; RNA(LIP); Tetravalent RNA-lipoplex Cancer Vaccine |
|
|
mRNA-4157/V940 |
Phase II |
NCT03897881 |
Active |
Malignant Melanoma |
Moderna Therapeutics. (Collaborator: Merck Sharp & Dohme LLC) |
mRNA encoding neoantigens for a personalized cancer vaccine to patient mutanome; immunostimulant |
mRNA 4157; PCV; Personalized Cancer Vaccine - Moderna Therapeutics |
|
|
GSK1572932A |
Phase II |
NCT00849875 |
Terminated |
Melanoma |
GlaxoSmithKline |
mRNA encoding single TAA: MAGE-A3 |
Zastumotide; Astuprotimut-R; D1/3 MAGE-3 fusion protein; D1/3 MAGE-3 fusion protein SB MAGE-3; D1/3 MAGE-3 His; D1/3 MAGE-3 His fusion protein; GSK 1572932A; GSK 2132231A; GSK 249553; GSK1203486A; MAGE-A3; MAGE-A3 antigen specific cancer immunotherapeutic; MAGE-A3 ASCI; NSC 719274; SB 249553; SB MAGE-3; SID 534984 |
|
|
Other RNAs |
||||||||
|
CV 8102 |
Phase I |
NCT03291002 |
Active |
Melanoma, Squamous Cell Carcinoma of the Skin |
CureVac AG (Collaborators: Syneos health and Cromos Pharma LLC) |
Non-coding, non-capped ssRNA (CV8102) complexed with a cationic peptide to induce an immune response via TLR-7/8 and DDX58/RIG-1; immunostimulant |
CV8102 |
|
|
BO 112 |
Phase II |
NCT04570332 |
Active |
Malignant melanoma |
Highlight therapeutics |
non-coding double stranded synthetic RNA activating TLR3, RIG-1, and MDA5 to sensitize tumor cells to immune response |
BO-112; Nanoplexed Poly IC BO-112; Nanoplexed Polyinosinic:Polycytidylic Acid BO-112 |
|
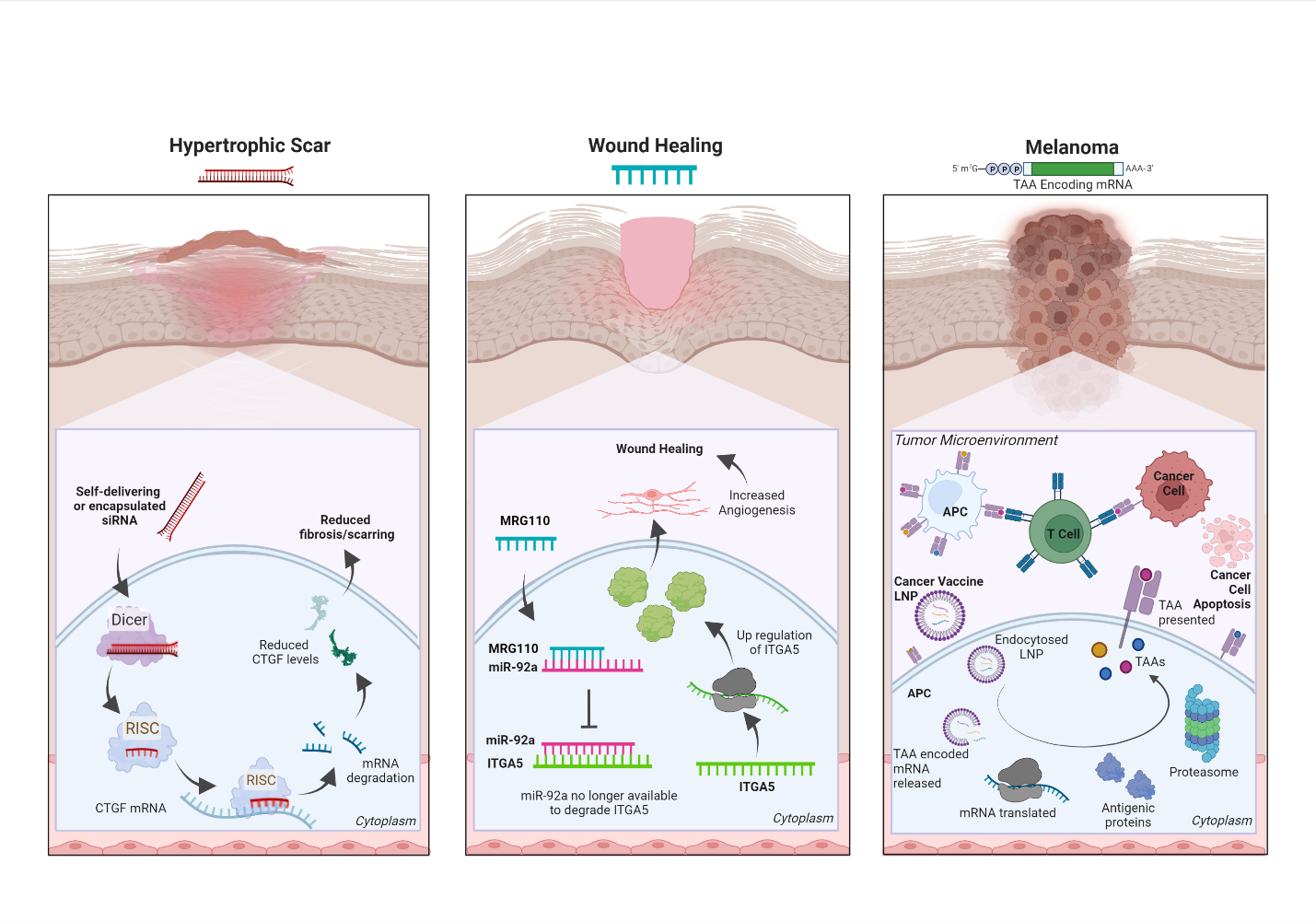
Figure 1: The mechanism of action for three RNA-based therapeutics. (left) Schematic depiction of the mechanism of action for multiple siRNAs currently in clinical trials for hypertrophic scars. All three siRNAs aim to reduce fibrosis and scarring by targeting CTGF mRNA to ultimately lower CTGF protein expression. (Center) The wound-healing candidate drug ASO MRG-110 directly targets miR-92a which represses ITGA5 a pro-angiogenic factor. Blocking the actions of miR-92a increases ITGA5 mRNA and protein levels to promote angiogenesis and wound healing. (Right) The mechanism of action depicting a mRNA-based cancer vaccine (e.g., BNT111) where a pool of mRNA encoding tumor-associated antigens (TAAs), linked to a specific cancer (i.e., BNT111 encodes 4 melanoma antigens), is delivered to antigen-presenting cells (APC) via lipid nanoparticles (LNP). The mRNA pool is translated into TAAs and elicits a specific host anti-tumoral immune response against the cancer cells. Created with BioRender.com.
RNA Interference (RNAi)
The aberrant expression of a protein, a mutated protein, or a non-coding RNA 3,4 can all cause skin disorders, and blocking such elements can be a beneficial therapeutic approach. RNA interference (RNAi) is one method to specifically target and lower the expression of pathological proteins. In RNAi, short double-stranded RNAs of exogenous or endogenous origins are processed by DICER and loaded into the RNA-induced silencing complex (RISC). Loaded RISC complexes recognize targeted mRNAs via perfect (siRNAs) or imperfect (miRNAs) complementarity and inhibit their translation and/or cause their degradation, thereby reducing the level of the encoded protein 5-7. siRNAs are designed to specifically target a single mRNA, whereas miRNAs (naturally occurring or synthetic) often have a broader array of targets 7.
The discovery of RNAi has greatly contributed to our understanding of the role(s) of countless proteins and their potential pathogenicity. More importantly to clinicians, RNAi provided a pathway to target disease-causing proteins that were otherwise impossible to treat. As with other RNA platforms, RNA stability, poor tissue penetration, and off-tissue targeting remain challenges; however, modifications to RNA bases and/or backbones plus the development of a wide variety of delivery systems enabled the FDA-approval of multiple siRNA therapeutics 8. Although several siRNA and miRNA mimics are currently advancing in clinical trials, no RNAi therapies have yet been approved for skin-related diseases.
Currently, at least three siRNAs (BMT101 (OLX101A), LEMS401, and RXI-109) targeting the connective tissue growth factor (CTGF) are in clinical trials (Figure 1, left). High levels of CTGF have been linked to fibrotic disorders, and reducing CTGF levels is thought to be a viable approach for reducing cutaneous fibrosis 8-11. Further, CTGF is also an attractive target for treating keloids or hypertrophic scars, which occur in 40-70% of patients following surgery 12.
Antisense Oligonucleotides (ASOs)
Oligonucleotides are short chains of polymerized nucleotides, and antisense oligonucleotides (ASO) are short (generally 12-30 nucleotides) single-stranded synthetic nucleic acids whose reverse complement sequence allows for the targeting of specific RNA or DNA sequences 13-16. The use of oligonucleotides in clinical trials dates back to the late 1950s and early 1960s when methods to synthesize them were first established 17. Although tested for decades, Fomivirsen, the first-in-class ASO, was only approved by the FDA to treat cytomegalovirus-induced retinitis in 1998 18.
ASOs can reduce or restore protein expression, inhibit 5' cap formation, or alter the splicing of targeted mRNAs. ASOs commonly enter the cell via endocytic pathways and recognize their targeted mRNAs through Watson-Crick base pairing 19,20. ASOs predominantly function via two mechanisms. First, ASOs can employ an occupancy-mediated degradation mechanism by inducing the cell’s RNase H nuclease activity to degrade a targeted mRNA 21. Second, ASOs can use an occupancy-only model and function via steric hindrance. This strategy can up or down-regulate target transcripts by altering their splicing patterns or by masking protein docking sites 22.
ASOs are usually trafficked to late endosomes and lysosomes which accounts for their slow release 23. Notably, the phosphodiester backbones of unmodified ASOs are prone to endonuclease degradation resulting in comparatively short half-lives; however, chemical modifications can overcome these shortcomings 24,25. ASOs are grouped into three generations based on their chemical modifications. (1) First-generation ASOs often replace a non-bridging oxygen atom in the phosphate group with either an amine (phosphoramidites), a methyl group (methyl phosphonates), or a sulfate group (phosphorothioates). When compared to phosphodiester oligonucleotides or unmodified ASOs, first-generation ASOs can resist nucleases and have longer half-lives in plasma 24. However, mRNA targeting affinity is slightly reduced due to the decreased melting temperature of these ASOs 15. (2) Second-generation ASOs usually have alkyl modifications at the 2' position of the ribose which improves binding affinity, tissue uptake, and nuclease resistance, while leading to both longer in vivo half-lives and lower toxicity 26. (3) Third-generation ASOs use chemical modifications to increase their stability, nuclease resistance, and hybridization affinity to the targeted RNA. The most commonly used third-generation ASOs usually incorporate peptide nucleic acids (PNA), locked nucleic acids (LNA), and morpholino phosphoramidite (MF) modifications 27. Notably, many third-generation ASOs function by causing steric hindrance of ribosomal machinery or altering the splicing of its targeted RNA 28,29.
MRG-110 (Figure 1, center), a third generation LNA ASO developed by miRagen Therapeutics, Inc. (Boulder, CO), is currently in phase 1 clinical trial for wound healing. It blocks miR-92a, which then de-represses the integrin alpha 5 (ITGA5) gene 30. Higher levels of ITGA5 protein promotes angiogenesis which facilitates wound healing 31. Studies have shown significant results for MRG-110 reporting none or very low systemic toxicity and drug accumulation in distal tissues.
Messenger RNA (mRNA)
mRNAs are transient RNAs that encode a protein. Exogenous mRNAs were first used to elicit specific protein expression in vivo over three decades ago 32. Despite that initial success, nearly two decades passed until data were reported for the first clinical trial employing mRNA as a therapeutic 33. The development of mRNA-based therapeutics has seen a renaissance as the COVID-19 global pandemic demonstrated their versatility and power. The mRNA-based therapies currently undergoing clinical trials for dermatologically related diseases are listed in Table 1. Three main mRNA treatment modalities have emerged. First, cancer vaccines use mRNAs encoding tumor-specific antigens to stimulate a protective immune response 1,34. Second, replacement therapies use mRNAs to produce therapeutic proteins or to counteract the phenotypes of a defective gene/protein. Third, cell-based therapies use mRNA transfected into cells ex vivo, with these cells being re-introduced into the patient to modify a specific diseased phenotype/function 1,35. Despite the broad applicability of mRNAs, the constraints of this mini review restrict our discussion to mRNA cancer vaccines.
Therapeutic cancer vaccines generally encode tumor-associated antigens (TAAs), or unique markers expressed in cancerous, but not normal, cells. Targeting several TAAs in a single vaccine reduces the risk of tumor antigen escape as it triggers a broad immune response and it aids in the detection of poorly expressed antigens, thereby increasing the robustness of the vaccine and its antitumor response. BioNTech initially pursued a cancer vaccine targeting four melanoma-associated antigens in their phase 1 Lipo-MERIT monotherapy trial 36. While this clinical trial is still ongoing, BioNTech has used the Lipo-MERIT data to steer the development of BioNTech’s FixVac (BNT111) therapy for melanoma, which shows a promising safety profile and anti-tumor immune response 37. BioNTech’s FixVac mRNA therapeutic platform is a fixed set of mRNA-encoded TAAs known to be expressed in particular cancer types (e.g., melanoma), therefore, prompting a strong and precise immune response against the particular cancer (Figure 1, right). BNT111 was developed to treat patients with anti-PD-1-refractory/relapsed unresectable stage III or IV melanoma and is one of the most promising cancer immunotherapies in development 38-42 .
Cancerous cells are characterized by their rapid proliferation and expansion which often generates somatic mutations, each of which becomes a potentially targetable neoantigen. A patient’s specific tumor neoantigen profile (mutanome) could be analyzed and incorporated into personalized neoantigen-encoding mRNA vaccines. As neoantigen expression is restricted to tumor cells, this feature could easily be exploited to treat melanomas as they have a high mutation burden 43. Recently, Moderna Inc. and Merck & Co. released promising new data regarding their mRNA-based personalized (neoantigen) cancer vaccine, mRNA-4157/V940, for the treatment of melanoma. When the vaccine is administered in concert with Keytruda, a cancer immunotherapy, reports show a 44% reduction in the risk of recurrence or patient death when compared to Keytruda alone over a period of 1 year 39,43,44. These data are a major breakthrough in the field of RNA therapeutics, demonstrating the power of mRNA to “train” a patient’s immune system to recognize and attack their specific tumor mutanome, hopefully, translating into durable remission.
mRNA cancer vaccines have many advantages. First, mRNA-based modalities elicit a potent yet reliable immune response to selected antigens in vivo and are customizable to a patient’s particular neoantigen profile. Second, mRNA therapies are safe compared to viral vector vaccines as they do not incorporate into the host genome, thus avoiding the risk of insertional mutagenesis. Lastly, mRNA vaccines are relatively inexpensive and rapid to synthesize 34. Altogether, mRNA therapeutics hold great promise for the treatment of various dermatological diseases.
Summary
As mentioned above, RNA therapeutics are a rapidly evolving class of therapies that have the potential to change the face of personalized medicine and revolutionize healthcare1. A number of RNA therapeutics have been approved by FDA and many more are in clinical trials for a broad array of indications. In this review, we focused exclusively on RNA therapeutics in clinical trials for dermatological disorders, such as those used to treat melanoma, psoriasis, hypertrophic scars, wound healing, alopecia, epidermolysis bullosa, keloids, etc. Our research uncovered 35 different RNA therapeutics currently undergoing clinical trials and dozens more are in the discovery or preclinical stages of development for skin conditions as well. In closing, many RNA therapies are rapidly progressing through clinical trials and this evolving and growing class of drug candidates offers much promise to improve existing treatment regimens or to become standalone treatments themselves.
Acknowledgments and Funding
REK, SK, and NB contributed equally to this work and all authors acknowledge that all three authors can list themselves first on career materials such as curriculum vitae. REK, SK, NB, and DLK all performed the literature searches and wrote and revised sections of the manuscript. REK, SK, and NB constructed and edited the table and figure. DLK reviewed and edited the entire manuscript incorporating revisions from all authors. All authors approved the final manuscript. All authors apologize for primary works that were not cited due to citation limits for minireviews. This work was supported by a Houston Methodist Research Institution (HMRI) Career Cornerstone Award, an award from the Houston Methodist Foundation (both to DLK) and Cancer Prevention and Research Institute of Texas (RP150611 and RP200619) grants to the RNA Core at the HMRI. DLK is supported in part by an R35 grant from the NIH (R35GM137819). REK is supported by an NIH postdoctoral supplement (R35GM137819-03S3). The content presented here is solely the responsibility of the authors and does not represent the official views of the HMRI, CPRIT, or the NIH.
Conflict of Interest
Dr. Kiss runs an externally funded laboratory (American Heart Association [20CDA35310329], and NIH [R35GM137819-03, -03S1, -03S2, -03S3], plus two subcontracts to NIH Contract #: 75N93019C00045) that is actively designing and testing different candidate RNA therapeutics. All authors anticipate seeking appropriate intellectual property protection for promising candidates that emerge from the lab's work. Dr. Kiss and Dr. Bejar are named inventors on a recently filed patent for a novel RNA therapy platform. Dr. Kiss also has patents planned for several additional RNA therapeutics and an inducible stable HUVEC cell line. Dr. Bejar is named as a co-inventor on a patent filing for a candidate RNA therapeutic (no.17969496). Further, Dr. Kiss serves as an ad hoc consultant for multiple different for-profit companies and to the RNA Core at the Houston Methodist Research Institute. Dr. Kiss also reports private stock for BioLife Solutions Inc.
References
- Damase TR, et al. The Limitless Future of RNA Therapeutics. Front Bioeng Biotechnol 9, 628137, doi: 10.3389/fbioe.2021.628137 (2021).
- Kalal BS, Upadhya D, Pai VR. Chemotherapy Resistance Mechanisms in Advanced Skin Cancer. Oncol Rev 11, 326, doi:10.4081/oncol.2017.326 (2017).
- Li X. et al. Targeting microRNA for improved skin health. Health Sci Rep 4, e374, doi: 10.1002/hsr2.374 (2021).
- Banerjee J, Chan YC, Sen CK. MicroRNAs in skin and wound healing. Physiol Genomics 43, 543-556, doi: 10.1152/physiolgenomics.00157.2010 (2011).
- Bartel DP. Metazoan MicroRNAs. Cell 173, 20-51, doi: 10.1016/j.cell.2018.03.006 (2018).
- Hu B. et al. Therapeutic siRNA: state of the art. Signal Transduction and Targeted Therapy 5, 101, doi: 10.1038/s41392-020-0207-x (2020).
- Lam JK, Chow MY, Zhang Y, et al. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol Ther Nucleic Acids 4, e252, doi: 10.1038/mtna.2015.23 (2015).
- Zhu Y, Zhu L, Wang X, et al. RNA-based therapeutics: an overview and prospectus. Cell Death Dis 13, 644, doi: 10.1038/s41419-022-05075-2 (2022).
- Bejar N, Tat TT, Kiss DL. RNA Therapeutics: the Next Generation of Drugs for Cardiovascular Diseases. Curr Atheroscler Rep 24, 307-321, doi:10.1007/s11883-022-01007-9 (2022).
- Makino K. et al. Anti-connective tissue growth factor (CTGF/CCN2) monoclonal antibody attenuates skin fibrosis in mice models of systemic sclerosis. Arthritis Res Ther 19, 134, doi: 10.1186/s13075-017-1356-3 (2017).
- Kang S. et al. RNAi nanotherapy for fibrosis: highly durable knockdown of CTGF/CCN-2 using siRNA-DegradaBALL (LEM-S401) to treat skin fibrotic diseases. Nanoscale 12, 6385-6393, doi: 10.1039/c9nr10305h (2020).
- Carswell L, Borger J. Hypertrophic Scarring Keloids, <https://www.ncbi.nlm.nih.gov/pubmed/30725743> (2022).
- Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 19, 673-694, doi: 10.1038/s41573-020-0075-7 (2020).
- Crooke ST, Baker BF, Crooke RM, et al. Antisense technology: an overview and prospectus. Nat Rev Drug Discov 20, 427-453, doi: 10.1038/s41573-021-00162-z (2021).
- Quemener AM, et al. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip Rev RNA 11, e1594, doi: 10.1002/wrna.1594 (2020).
- Di Fusco D. et al. Antisense Oligonucleotide: Basic Concepts and Therapeutic Application in Inflammatory Bowel Disease. Front Pharmacol 10, 305, doi: 10.3389/fphar.2019.00305 (2019).
- Reese CB. The chemical synthesis of oligo-and poly-nucleotides by the phosphotriester approach. Tetrahedron 34, 3143-3179 (1978).
- Orr RM. Technology evaluation: fomivirsen, Isis Pharmaceuticals Inc/CIBA vision. Curr Opin Mol Ther 3, 288-294 (2001).
- Chan JH, Lim S, Wong WS. Antisense oligonucleotides: from design to therapeutic application. Clin Exp Pharmacol Physiol 33, 533-540, doi: 10.1111/j.1440-1681.2006.04403.x (2006).
- Juliano RL, Carver K. Cellular uptake and intracellular trafficking of oligonucleotides. Adv Drug Deliv Rev 87, 35-45, doi: 10.1016/j.addr.2015.04.005 (2015).
- Liang XH, Sun H, Nichols JG. RNase H1-Dependent Antisense Oligonucleotides Are Robustly Active in Directing RNA Cleavage in Both the Cytoplasm and the Nucleus. Mol Ther 25, 2075-2092, doi: 10.1016/j.ymthe.2017.06.002 (2017).
- Desterro J, Bak-Gordon P, Carmo-Fonseca M. Targeting mRNA processing as an anticancer strategy. Nat Rev Drug Discov 19, 112-129, doi: 10.1038/s41573-019-0042-3 (2020).
- Juliano RL. The delivery of therapeutic oligonucleotides. Nucleic Acids Res 44, 6518-6548, doi: 10.1093/nar/gkw236 (2016).
- Younis HS, et al. in A Comprehensive Guide to Toxicology in Preclinical Drug Development (ed Ali S. Faqi) Ch. 26, 647-664 (Academic Press, 2013).
- Wan J, Bauman JA, Graziewicz MA, et al. Oligonucleotide therapeutics in cancer. Cancer Treat Res 158, 213-233, doi: 10.1007/978-3-642-31659-3_9 (2013).
- Agrawal S. et al. Mixed-backbone oligonucleotides as second generation antisense oligonucleotides: in vitro and in vivo studies. Proc Natl Acad Sci U S A 94, 2620-2625, doi: 10.1073/pnas.94.6.2620 (1997).
- Sardone V, Zhou H, Muntoni F, et al. Antisense Oligonucleotide-Based Therapy for Neuromuscular Disease. Molecules 22, doi: 10.3390/molecules22040563 (2017).
- Wahlestedt C, et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc Natl Acad Sci U S A 97, 5633-5638, doi: 10.1073/pnas.97.10.5633 (2000).
- Havens MA, Hastings ML. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res 44, 6549-6563, doi: 10.1093/nar/gkw533 (2016).
- Gallant-Behm CL, et al. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair Regen 26, 311-323, doi: 10.1111/wrr.12660 (2018).
- Huang CK, Kafert-Kasting S, Thum T. Preclinical and Clinical Development of Noncoding RNA Therapeutics for Cardiovascular Disease. Circ Res 126, 663-678, doi: 10.1161/CIRCRESAHA.119.315856 (2020).
- Wolff JA, et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465-1468, doi: 10.1126/science.1690918 (1990).
- Weide B, et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother 31, 180-188, doi: 10.1097/CJI.0b013e31815ce501 (2008).
- Gu Y, Duan J, Yang N, et al. mRNA vaccines in the prevention and treatment of diseases. MedComm (2020) 3, e167, doi: 10.1002/mco2.167 (2022).
- van Dulmen M, Rentmeister A. mRNA Therapies: New Hope in the Fight against Melanoma. Biochemistry 59, 1650-1655, doi: 10.1021/acs.biochem.0c00181 (2020).
- ClinicalTrials.gov. <https://clinicaltrials.gov/ct2/show/NCT02410733?cond=NCT02410733&draw=2> (2022a).
- Sahin U, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107-112, doi:10.1038/s41586-020-2537-9 (2020).
- ClinicalTrials.gov. <https://clinicaltrials.gov/> (2022b).
- AdisInsight. <https://adisinsight.springer.com/> (2023).
- BioNTech. <https://investors.biontech.de/news-releases/news-release-details/biontech-receives-fda-fast-track-designation-its-fixvac> (2021).
- BioNTech. <https://www.biontech.com/int/en/home/pipeline-and-products/platforms/our-mrna-platforms.html> (2023).
- clinicaltrials.gov. <https://clinicaltrials.gov/ct2/show/NCT04526899 > (2022c).
- Moderna. <https://investors.modernatx.com/news/news-details/2022/Moderna-and-Merck-Announce-mRNA-4157V940-an-Investigational-Personalized-mRNA-Cancer-Vaccine-in-Combination-with-KEYTRUDAR-pembrolizumab-Met-Primary-Efficacy-Endpoint-in-Phase-2b-KEYNOTE-942-Trial/default.aspx#> (2022).
- Vitale G. Chemical & Engineering News, <https://cen.acs.org/pharmaceuticals/vaccines/ModernaMerck-cancer-vaccine-shows-promise/100/web/2022/12?utm_source=LatestNews&utm_medium=LatestNews&utm_campaign=CENRSS> (2023).
