The 2022 Global Monkeypox Outbreak: A Focused Review
Yasmine Oprea1, Patricia Cerri-Droz2, Urmi Khanna1
1 Albert Einstein College of Medicine/Montefiore Medical Center
2 Renaissance School of Medicine at Stony Brook University
Abstract
The first human infection with monkeypox virus was reported in 1970, and infections have subsequently been recorded in endemic areas such as Central and West Africa or linked to international travel to these regions. However, the emergence of the 2022 monkeypox outbreak has involved multiple non-endemic countries and continents without links to travel to endemic areas. The first cases in the current outbreak were reported in May of 2022. The primary mode of transmission is atypical and is thought to occur through direct contact with infected skin lesions. The rapid increase in case numbers prompted the World Health Organization to declare this disease outbreak as a public health emergency of international concern. Robust efforts are being made by global public health authorities to develop effective antiviral treatment options and vaccination strategies to reduce the spread of this disease. The objective of this manuscript is to provide a comprehensive review of the 2022 mpox outbreak with respect to its unique epidemiology, clinical features, complications, and management options.
Introduction
Monkeypox is a zoonotic disease caused by monkeypox virus, an orthopoxvirus member of the Poxviridae family that has been declared a public health emergency due to global outbreaks occurring in non-endemic areas1,2. In November 2022, the World Health Organization (WHO) announced that “mpox” would be used as a preferred term instead of monkeypox due to racist and stigmatizing language associated with the disease. There are two known clades of the Monkeypox virus: the Central African (Congo Basin) clade, now referred to as Clade I, and the West African clade, now known as Clade II. Clade II is subdivided into two subgroups, known as IIa and IIb. Evolutionary divergence between the two clades has resulted in nucleotide variations that have led to differences in the viral proteins responsible for viral persistence and immune system evasion. Thus, phylogenetic and clinical differences distinguish the Clade I virus from the Clade II virus2,3. Clade I is responsible for causing more severe illness, but Clade II has demonstrated higher rates of human-to-human transmission, accounting for most of the cases in the current outbreak1-3.
Epidemiology
The CDC estimates that as of December 2022, there have been approximately 82,200 confirmed cases reported worldwide, with over 98% of those reported in non-endemic regions, including North and South America, Europe, and Asia. The United States (US) makes up over 29,000 of those cases. New infections in the US appeared to reach a peak in the summer months (July-August) of 2022, reaching a 7-day average of over 400 confirmed cases, and have been down trending in the recent months1,2.
Human-to-human transmission occurs via contact with contaminated bodily fluids, materials such as clothing or bedding, or skin lesions of an infected person4. Initially, it was postulated that this virus can spread through respiratory droplets, but subsequent research has shown that the viral load in oropharyngeal swabs was approximately 17-fold less than in swabs of skin lesions, making this a less likely route of transmission5. Cases have been disproportionately reported in communities of men identifying as having sex with men (MSM), where close skin-to-skin contact is hypothesized to facilitate transmission of the virus. Women and children are less commonly affected1,6.
Clinical Features
The mean incubation time for monkeypox virus from contact to symptom onset was found to be between 6 and 9 days in recent studies7. Classically, the first symptoms of mpox infection involve a nonspecific prodrome that most commonly includes fever, fatigue, myalgia, and lymphadenopathy8. The current outbreak of mpox has presented with atypical clinical characteristics, with many patients facing symptoms deviating from those previously described7. Most notably, there is frequent absence of the prodromal symptoms of fever, headache, adenopathy, and malaise before cutaneous involvement9,10. Additionally, emerging data suggests that a large proportion of mpox infections are transmitted during the pre-symptomatic period, from 1 to 4 days before the appearance of any initial symptoms7.
One to five days following the prodrome, an eruption of painful vesiculopustular lesions of variable sizes is typically noted (Figure 1). The location of the rash is often dependent on the site of infection. The rash may present as macules, papules, or vesicles in the early stage (day 1-2 following the prodrome) which then evolve into firm pustules (day 7) and resolve into scabs with crusting. These lesions can be found on the face, hands/feet, and oral mucosa, and if present in multiple areas of the body, they often present simultaneously and in the same stage8,11,12. Though recent presentation has predominated in areas of sexual contact, such as the anogenital or the oral regions, it remains unknown whether the infection is sexually transmitted9,10,13. It is also unclear why some patients may present with disseminated monkeypox infection whereas infection in others is limited to one area of the body.
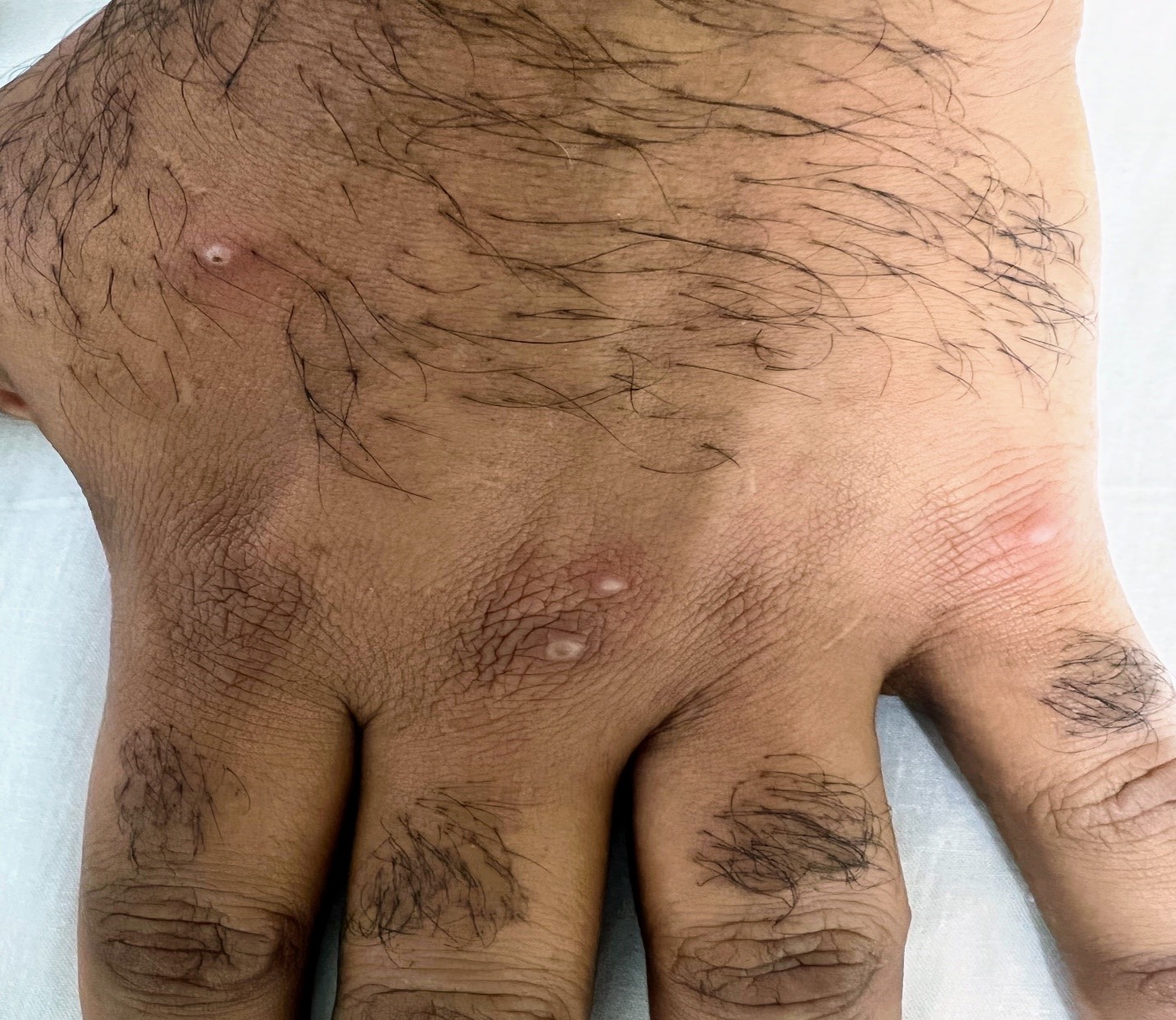
Figure 1: A young male presenting with discrete vesicles and pustules with central umbilication on the left dorsal hand. A polymerase chain reaction was positive for non-variola orthopox virus.
The patient is considered infectious until there is re-epithelialization of all open vesiculopustular lesions on the skin11,14. In addition to the skin findings, features of the mpox enanthem observed during this outbreak include pharyngitis, myocarditis, epiglottitis, as well as proctitis or anorectal pain.
The lesions caused by the monkeypox virus may appear similar to other infectious vesiculopustular lesions, so multiple differential diagnoses may be considered when a patient presents with skin findings concerning for mpox. In particular, mpox lesions must be differentiated from the erythematous vesiculopustular lesions seen in smallpox infection, and other infections such as varicella, disseminated herpes simplex virus, coxsackie virus, and molluscum contagiousum. Disseminated herpes simplex infections can be distinguished by smaller monomorphic lesions. Coxsackievirus infections affect palmoplantar surfaces, presenting with football-shaped vesiculobullous lesions. Most notably, molluscum contagiosum and smallpox exhibit similar vesiculopustular lesions found in mpox that are difficult to deroof and often do not display fluid contents11. However, unlike the clustered vesicles found in these diseases, mpox lesions appear larger, as well as more isolated, umbilicated, and crateriform8.
Diagnostic Testing
The WHO and Centers for Disease Control and Prevention (CDC) recommend that all individuals who present with rash and fever or lymphadenopathy be screened for suspected mpox infection15,16. Clinicians should refer to diagnostic guidelines set forth by the CDC and WHO when addressing diagnosis. The confirmation of an mpox infection may be reached following detection of non-variola orthopox DNA through real-time polymerase chain reaction (RT-PCR) and is further distinguished through monkeypox-specific RT-PCR testing or sequencing of specimens retrieved from the individual17. Specimens for such tests may be collected through vigorous dry swabbing of lesions in the oropharynx, anorectal region, or other areas of disease. Importantly, de-roofing is not essential. Although not readily indicated, Tzanck smears may allow for the rapid cytological interpretation and ruling out of other conditions that present similarly, such as molluscum contagiosum and herpes virus infection17. Per the recommendation of the CDC, culturing monkeypox specimens for the purpose of diagnosis should not be performed for routine diagnosis16. While performing these diagnostic tests, personal protective equipment and careful collection of samples must be prioritized to avoid autoinoculation or transmission of the virus.
Management
Most mpox infections are mild and resolve without specific therapies within 2-4 weeks following infection11. Primary treatment upon hospitalization has consisted of supportive management of mucosal lesion pain, proctitis, and mitigation of secondary infections of the skin18,19. Considerations for general pain control include over-the-counter medications (OTC) such as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs). Oropharyngeal pain may be managed with rinses of viscous lidocaine along with oral antiseptics to suppress bacterial infection15. Treatment of proctitis associated with this infection is challenging. Along with OTC medications, sitz baths and application of topical analgesics such as lidocaine may provide some relief.
Antiviral medications approved for treatment of smallpox, including tecovirimat and brincidofovir, may be useful in those at risk of severe disease manifestation, such as children, immunocompromised individuals, and pregnant women11.
Tecovirimat is the first antiviral treatment approved by the US Food and Drug Administration (FDA) for both pediatric (≥13 kg) and adult smallpox, taken orally at varying doses depending upon body weight for 14 days20. Orthopoxvirus viral envelope protein VP37 is inhibited by tecovirimat, hindering viral release from infected cells and thus further viral dissemination21. Its use has been allowed through the Expanded Access Investigational New Drug application (EA-IND) for potential treatment of other orthopoxviruses such as monkeypox. Although there has been relatively little administration of antiviral therapy during the current outbreak, a few cases of immunocompromised persons have noted disease resolution upon receiving tecovirimat20. However, experimental data have suggested that tecovirimat may have a low barrier to resistance, and there have been emerging reports of tecovirimat-resistant mpox cases22. Of the two cases of tecovirimat resistance first reported by the CDC, both patients were immunocompromised and had progressive and disseminated mpox infection that was not adequately controlled by prolonged treatment with tecovirimat. A proposed mechanism for tecovirimat-resistance is due to F13L mutations in the viral genome22.
Brincidofovir is an FDA-approved antiviral medication indicated for the treatment of pediatric (≥10 kg) and adult smallpox, working through the inhibition of viral DNA polymerase. It remains under consideration for mpox treatment under EA-IND as an oral analog of intravenous cidofovir, as it causes lower hepatic and renal toxicity20,23. Similar to tecovirimat, the effects of brincidofovir have not yet been well-documented apart from numbered reports of resolved disease following oral administration of varying doses and frequency23.
Although the safety of tecovirimat and brincidofovir has been confirmed through human studies, their efficacy has been proved solely in animal models and few patients23,24. Therefore, antiviral use in treating human mpox remains under investigation20. Ongoing studies are needed to provide more evidence for the efficacy of these antiviral therapies in mpox.
An additional therapy for mpox infection may be intravenous Vaccinia Immune Globulin, previously approved for the treatment of vaccinia vaccination complications such as eczema vaccinatum and progressive vaccinia8. Such treatment is practical for immunocompromised individuals unable to receive vaccination, and is under consideration for EA-IND.
Individuals who suspect exposure to mpox should monitor their symptoms in the 16-to-23-day period following possible exposure, observing for the appearance of rash, lesions, or the onset of fever or lymphadenopathy7,16. Patients who are actively infected and presenting with mild disease should remain isolated at home until all the lesions have crusted over and the scabs have fallen off with complete re-epithelization16.
In addition to monitoring symptoms and self-isolation following a suspected mpox encounter, the CDC provides additional guidelines to control and prevent the spread of the monkeypox virus. It is suggested to avoid direct skin-to-skin contact with individuals who have a rash that appears to be mpox, whether it appears on the genitals, trunk, or extremities25. Additionally, avoiding contact with fomites such as utensils, clothing, bedding, or towels of a person with suspected or confirmed mpox virus is also recommended. Finally, prevention strategies such as proper hand hygiene and vaccination are strongly recommended to protect against mpox infection and spread, especially given the emergence of evidence supporting high prevalence of pre-symptomatic transmission7,25. Given the risk of transmissibility through both contact and aerosolization, especially in healthcare settings, it is imperative for healthcare workers to don proper personal protective equipment (PPE) and to use the Identify-Isolate-Inform (3I) tool to manage suspected mpox cases in clinical settings26.
Vaccination
Vaccination is recommended for individuals with known or presumed exposure to monkeypox or those classified as high risk for occupational exposure, such as healthcare personnel, laboratory workers, and veterinarians. Two vaccines, JYNNEOS and ACAM2000 (Table 1), have been indicated for pre-exposure prophylaxis with 85% efficacy, as well as post-exposure prophylaxis if administered promptly following close contact8,11,27.
Table 1: Comparison of Currently Available Vaccination Options for Monkeypox
|
Vaccine Name |
JYNNEOS |
ACAM2000 |
|
Vaccine Type |
Live, replication-incompetent modified vaccinia virus Ankara vaccine |
Live, replication-competent vaccinia virus vaccine |
|
Regimen of Vaccination |
Two dose series, 28 days apart |
One dose |
|
Route of Administration |
I.Intradermal injection in the forearm of 0.1 mLa II.Subcutaneous injection in the upper arm of 0.5 mLb |
Percutaneous scarification of the skin with 0.0025 mL droplet (multiple punctures of skin with a bifurcated needle containing vaccinia virus) |
|
Recommended Population |
I.Intradermal injection (Alternative): < 18 years of age II.Subcutaneous injection: >18 years of age, or at risk of developing keloid scars |
Greater than 12 months of age |
|
Contraindications |
Persons with history of severe allergic reactions to vaccine ingredients |
Pregnancy; persons with history of severe allergic reactions, or atopic dermatitis, eczema, psoriasis and exfoliative skin disorders; infants; Immunocompromised individuals (HIV, leukemia, transplant recipients); heart disease |
|
Adverse Reactions |
Injection site swelling, pain, and redness. |
Injection site swelling, pain, and redness; lymphadenopathy, itching, malaise, fatigue; eczema vaccinatum, progressive vaccinia (rare)c |
a The alternative regimen is an injection volume of 0.1 mL intradermally for those greater than 18 years of age, decreasing the dosage to improve vaccine availability during the outbreak28.
b The standard regimen of 0.5 mL subcutaneous administration is recommended for individuals less than 18 years of age, or those at risk of developing keloid scars28.
c In immunocompromised persons, the vaccine may generate uncontrolled viral shedding at the site of inoculation, leading to progressive vaccinia, a rare adverse event associated with an expanding and necrotic vaccination site. Those with a history of atopic dermatitis may present with eczema vaccinatum, a complication in which the vaccinia virus exploits the already weakened skin-barrier for replication29.
ACAM2000 is a live and replication-competent vaccinia virus vaccine, approved by the FDA in 2007 for the prevention of smallpox. Recently, it has also been approved for the prevention of mpox under EA-IND30. Orthopoxviruses, such as monkeypox, vaccinia virus, and Variola virus (smallpox), feature a highly conserved genome, allowing for orthopoxvirus infection or immunization with an orthopoxvirus vaccine to provide a level of immunity against other viruses within the genus31. One-dose ACAM2000 has mitigated previous monkeypox outbreaks, demonstrating the cross-reactive immunity potential of the vaccine32. Despite this capability, the vaccinia vaccine has been shown to elicit several unfavorable reactions among inoculated individuals and their close contacts, due in part to its replication competence27.
JYNNEOS is a live and replication-incompetent modified vaccinia virus Ankara vaccine authorized in September 2019 by the FDA for the treatment of smallpox. In August 2022, an Emergency Use Authorization issued by FDA approved the vaccination for mpox28. However, there have been reports of injection site pain, redness, and swelling18. Unlike ACAM2000, extensive trials regarding adverse events have yet to be conducted27, which is another area that requires additional research.
Post-exposure prophylaxis with these vaccinations holds potential due to the more drawn-out incubation period of mpox6. Onset of symptoms usually occurs several days after exposure; thus vaccination is recommended 4 days after known exposure to prevent disease or within 4-14 days to lessen the symptoms of infection7,11.
Complications
Mortality due to monkeypox virus remains low, at an estimated 65 total deaths worldwide according to the CDC in December of 20221. The most common complications of this infection may cause significant morbidity, including bacterial superinfection of skin lesions, sepsis, permanent skin scarring, and residual hypo- or hyperpigmentation of the skin33. Eczema monkeypoxium is a newly identified phenomenon thought to result from predisposition to monkeypox infection due to impairments in the skin barrier secondary to preexisting atopic dermatitis. However, the long-term sequela of monkeypox virus superinfection on atopic dermatitis are not yet fully understood34. Systemic complications such as neurologic, ocular, and pulmonary symptoms have also been emerging throughout the current outbreak. Pulmonary complications are thought to result from inflammation caused by the virus in the lung tissue, most commonly leading to bronchopneumonia2. The most commonly reported neurologic complications include headache and mood disturbances, but few cases of severe complications such as encephalitis have been reported35. Ocular manifestations during this outbreak have also been reported to cause morbidity on a spectrum, ranging from conditions such as conjunctivitis and keratitis to more severe complications such as corneal ulcers and scarring36.
Conclusions
Monkeypox (mpox) remains a global health concern, highlighting the need for continued vigilance and efforts to halt community transmission. Even as reported mpox cases continue to decline, it is imperative for clinicians to remain updated regarding the varied presentations of this viral disease, its clinical mimickers, and guidelines regarding use of antivirals and vaccines while managing this condition. Further research in multiple areas is needed to better understand the transmission of the monkeypox virus during this current outbreak and efficacy of existing antiviral therapies.
Conflict of Interest Statement:
The authors declare no conflicts of interest.
Funding Information:
No funding sources.
References
- CDC. United States monkeypox response and recommendations, 2022. Accessed December 8, 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/index.html.
- World Health Organization Disease Outbreak News; Multi-country monkeypox outbreak in non-endemic countries, 2022. Accessed December 8, 2022. https://www.who.int/emergencies/ disease-outbreak-news/item/2022-DON388.
- Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005 Oct; 86(Pt 10): 2661-2672. https://doi.org/10.1099/vir.0.81215-0.
- Alakunle E, Moens U, Nchinda G, et al. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses. 2020; 12(11): 1257. https://doi.org/10.3390/v12111257.
- Paran N, Yahalom-Ronen Y, Shifman O, et al. Monkeypox DNA levels correlate with virus infectivity in clinical samples, Israel, 2022. Euro Surveill. 2022 Sep; 27(35): 2200636. https://doi.org/10.2807/1560-7917.ES.2022.27.35.2200636.
- Khanna U, Bishnoi A, Singh K, et al. Clinical Considerations in Pediatric Cases of Monkeypox. J Am Acad Dermatol. 2022 Sep 13: S0190-9622(22)02686-X. doi: 10.1016/j.jaad.2022.09.009. Epub ahead of print. PMID: 36113613.
- Ward T, Christie R, Paton R S, et al. Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ 2022; 379: e073153. https://doi.org/10.1136/bmj-2022-073153.
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: A historical review and dermatologic primer. J Am Acad Dermatol. 2022 Nov; 87(5): 1069-1074. doi: 10.1016/j.jaad.2022.07.007. Epub 2022 Jul 8. PMID: 35817333; PMCID: PMC9528236.
- Otu A, Ebenso B, Walley J, et al. Global human monkeypox outbreak: atypical presentation demanding urgent public health action. Lancet Microbe. 2022 Aug; 3(8): e554-e555. doi: 10.1016/S2666-5247(22)00153-7. Epub 2022 Jun 7. PMID: 35688169; PMCID: PMC9550615.
- Huang ST, Wu YH, Lin HH, et al. The first imported case of monkeypox in Taiwan. J Formos Med Assoc. 2022 Sep 26: S0929-6646(22)00324-2. doi: 10.1016/j.jfma.2022.08.014. Epub ahead of print. PMID: 36175217; PMCID: PMC9534171.
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox: Essentials for the dermatologist. J Am Acad Dermatol. 2022 Nov; 87(5): e171-e174. doi: 10.1016/j.jaad.2022.06.1170. Epub 2022 Jun 24. PMID: 35753551; PMCID: PMC9528187.
- Petersen E, Kantele A, Koopmans M, et al. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect Dis Clin North Am. 2019 Dec; 33(4): 1027-1043. doi: 10.1016/j.idc.2019.03.001. Epub 2019 Apr 11. PMID: 30981594; PMCID: PMC9533922.
- Eisenstadt R, Liszewski WJ, Nguyen CV. Recognizing Minimal Cutaneous Involvement or Systemic Symptoms in Monkeypox. JAMA Dermatol. 2022 Oct 6. doi: 10.1001/jamadermatol.2022.4652. Epub ahead of print. PMID: 36201177.
- Adler H, Gould S, Hine P, et al; NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022 Aug; 22(8): 1153-1162. doi: 10.1016/S1473-3099(22)00228-6. Epub 2022 May 24. Erratum in: Lancet Infect Dis. 2022 Jul; 22(7): e177. Erratum in: Lancet Infect Dis. 2022 Jul;22(7): e177. PMID: 35623380; PMCID: PMC9300470.
- World Health Organization. (2022, 10 June.). Clinical management and infection prevention and control for Monkeypox: Interim rapid response guidance, 10 June 2022. World Health Organization. Retrieved November 4, 2022, from https://www.who.int/publications-detail-redirect/WHO-MPX-Clinical-and-IPC-2022.1.
- Centers for Disease Control and Prevention. (2022, September 7). Monkeypox. Centers for Disease Control and Prevention. Retrieved November 4, 2022, from https://www.cdc.gov/poxvirus/monkeypox/index.html.
- Khanna U, Kost Y, Wu B. Diagnostic considerations in suspected cases of monkeypox. J Am Acad Dermatol. 2022 Sep 24: S0190-9622(22)02777-3. doi: 10.1016/j.jaad.2022.09.034. Epub ahead of print. PMID: 36167184; PMCID: PMC9534272.
- Sah R, Mohanty A, Hada V, et al. The Emergence of Monkeypox: A Global Health Threat. Cureus. 2022 Sep 18; 14(9): e29304. doi: 10.7759/cureus.29304. PMID: 36277578; PMCID: PMC9579419.
- Thornhill JP, Barkati S, Walmsley S, et al. SHARE-net Clinical Group. Monkeypox Virus Infection in Humans across 16 Countries - April-June 2022. N Engl J Med. 2022 Aug 25; 387(8): 679-691. doi: 10.1056/NEJMoa2207323. Epub 2022 Jul 21. PMID: 35866746.
- Center for Drug Evaluation and Research. (2018, June 13). FDA approves the first drug with an indication for treatment of smallpox. U.S. Food and Drug Administration. Retrieved October 28, 2022, from https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-indication-treatment-smallpox.
- Russo AT, Grosenbach DW, Chinsangaram J, et al. An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. Expert Rev Anti Infect Ther. 2021 Mar; 19(3): 331-344. doi: 10.1080/14787210.2020.1819791. Epub 2020 Sep 15. PMID: 32882158; PMCID: PMC9491074.
- “Han Archive - 00481.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 4 Nov. 2022, https://emergency.cdc.gov/han/2022/han00481.asp#. Accessed December 8th, 2022.
- Kuroda N, Shimizu T, Hirano D, et al. Lack of clinical evidence of antiviral therapy for human monkeypox: A scoping review. J Infect Chemother. 2022 Oct 22: S1341-321X(22)00292-6. doi: 10.1016/j.jiac.2022.10.009. Epub ahead of print. PMID: 36283609; PMCID: PMC9598785.
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral Tecovirimat for the Treatment of Smallpox. N Engl J Med. 2018 Jul 5; 379(1): 44-53. doi: 10.1056/NEJMoa1705688. PMID: 29972742; PMCID: PMC6086581.
- Centers for Disease Control and Prevention. (2022, October 31). How to Protect Yourself. Centers for Disease Control and Prevention. Retrieved December 8, 2022, from https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html.
- Koenig KL, Beÿ CK, Marty AM. Monkeypox 2022 Identify-Isolate-Inform: A 3I Tool for frontline clinicians for a zoonosis with escalating human community transmission. One Health. 2022 Dec; 15: 100410. https:/doi.org/10.1016/j.onehlt.2022.100410. Epub 2022 Jun 24.
- Rizk JG, Lippi G, Henry BM, et al. Prevention and Treatment of Monkeypox. Drugs. 2022 Jun; 82(9): 957-963. doi: 10.1007/s40265-022-01742-y. Epub 2022 Jun 28. Erratum in: Drugs. 2022 Aug; 82(12): 1343. PMID: 35763248; PMCID: PMC9244487.
- Centers for Disease Control and Prevention. (2022, October 21). JYNNEOS vaccine. Centers for Disease Control and Prevention. Retrieved October 28, 2022, from https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/jynneos-vaccine.html#toc.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008 May 15; 46(10): 1555-61. doi: 10.1086/587668. PMID: 18419490.
- Centers for Disease Control and Prevention. (2022, October 21). ACAM2000 vaccine. Centers for Disease Control and Prevention. Retrieved October 29, 2022, from https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/acam2000-vaccine.html.
- Erratum: Vol. 71, No. 22. MMWR Morb Mortal Wkly Rep 2022; 71: 886. doi: http://dx.doi.org/10.15585/mmwr.mm7127a5.
- Ahmed SF, Sohail MS, Quadeer AA, et al. Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus. Viruses. 2022 Sep 3; 14(9): 1960. doi: 10.3390/v14091960. PMID: 36146766; PMCID: PMC9506226.
- Moore M, Zahra F. Monkeypox. In: StatPearls. StatPearls Publishing; 2022.
- Xia J, Huang CL, Chu P, et al. Eczema monkeypoxicum: Report of monkeypox transmission in a patient with atopic dermatitis. JAAD Case Rep. 2022 Aug 27; 29: 95-99. doi: 10.1016/j.jdcr.2022.08.034. PMID: 36212897; PMCID: PMC9534102.
- Billioux BJ, Mbaya OT, Sejvar J, et al. Neurologic Complications of Smallpox and Monkeypox: A Review. JAMA Neurol. Published online September 20, 2022. doi:10.1001/jamaneurol.2022.3491.
- V Mazotta, A Mondi, F Carletti, et al. Ocular involvement in monkeypox: Description of an unusual presentation during the current outbreak. J Infect 2022; 17: 1–3.
