Successful Use of Brentuximab Vedotin for Treatment of CD30-positive Primary Cutaneous Extranodal Natural Killer/T-Cell Lymphoma
Denise Ann Tsang1*, Chee Leong Cheng2, Laura Hui1
1Singapore General Hospital, Department of Dermatology
2Singapore General Hospital, Department of Pathology
Case Presentation
A 75-year-old Chinese man with a history of diabetes mellitus presented with multiple tender dermal violaceous ulcerative nodules over his extremities, which have increased in size and extent since their appearance 3 months ago (Figure 1). He reported a history of weight loss without fever or nasal symptoms. Histology from skin punch biopsies taken from his left arm and left thigh showed sheet-like infiltrate of medium-to-large atypical lymphoid cells. Immunohistochemistry (IHC) revealed positive staining with cytoplasmic CD3, CD2, CD56, CD30 and granzyme B. IHC staining for CD30 was variably weakly positive in a minority of the atypical lymphoid cells, ranging from less than 5% positivity in the left thigh specimen and 10-20% weak positivity in the left arm specimen, where atypical lymphoid cells appear larger. Ki-67 proliferation fraction was 90%. There was widespread nuclear positivity for Epstein-Barr virus-encoded small RNAs (EBER). IHC staining was negative for CD10, CD79a, CD 123 and TCL1 (Figures 2 and 3). A Positron Emission Tomography and Computed Tomography (PET-CT) showed multiple enhancing and hypermetabolic nodular skin lesions with no involvement of lymph nodes and other organs (Figure 4). He was diagnosed with high-risk, advanced stage primary cutaneous extranodal natural killer/T-cell lymphoma, nasal type (ENKTL-NT). He received upfront treatment with Brentuximab vedotin (BV) in addition to chemotherapy (Table 1) which resulted in complete response.
Table 1: Summary of Selected Studies
|
T: Brentuximab 1.8mg/kg T+2w: #1 GELOX. Developed acute pancreatitis to L-asparaginase, conservatively managed T+6w: #2 GELOX. Dose of L-asparaginase reduced by 50% T+9w: #3 GELOX. Developed anaphylactoid reaction to L-asparaginase. Decision made not for re-challenge T+13w: #1 mini-CHOP T+17w: #2 mini-CHOP T+21w: #3 mini-CHOP T+23w: Interval PET-CT showing resolution of all cutaneous nodules |
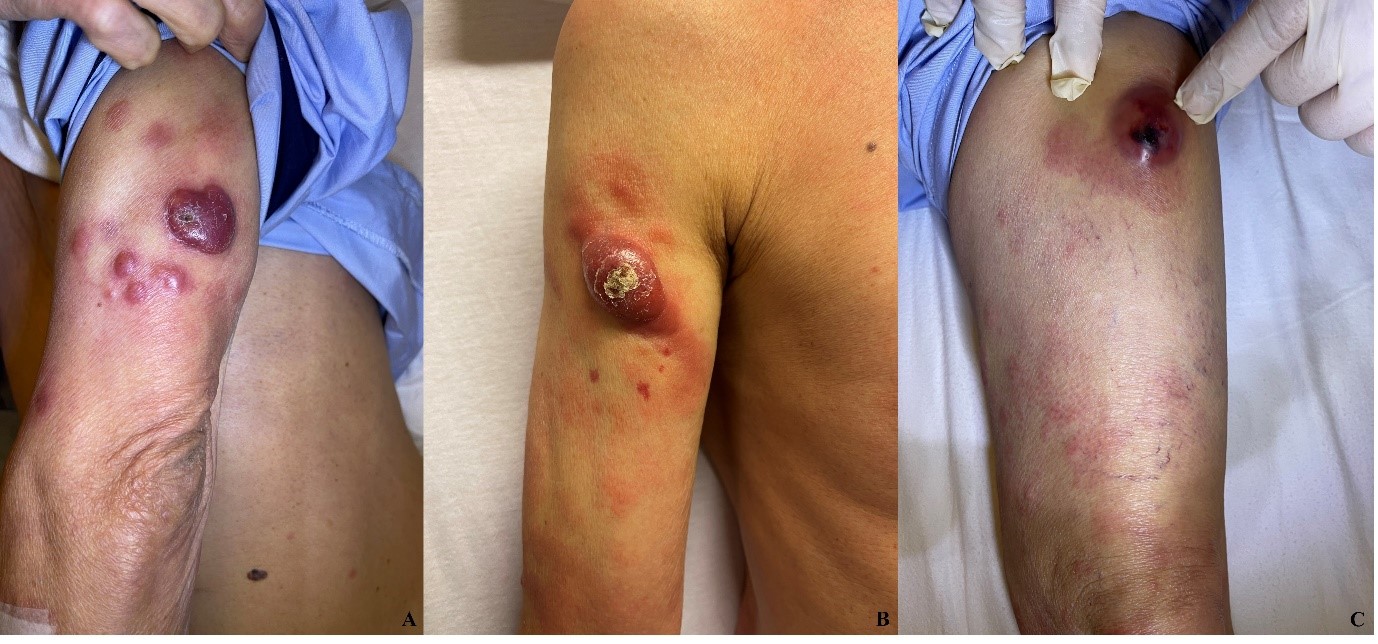
Figure 1: A. Left arm nodules, B. Right arm nodule, C. Left thigh nodule
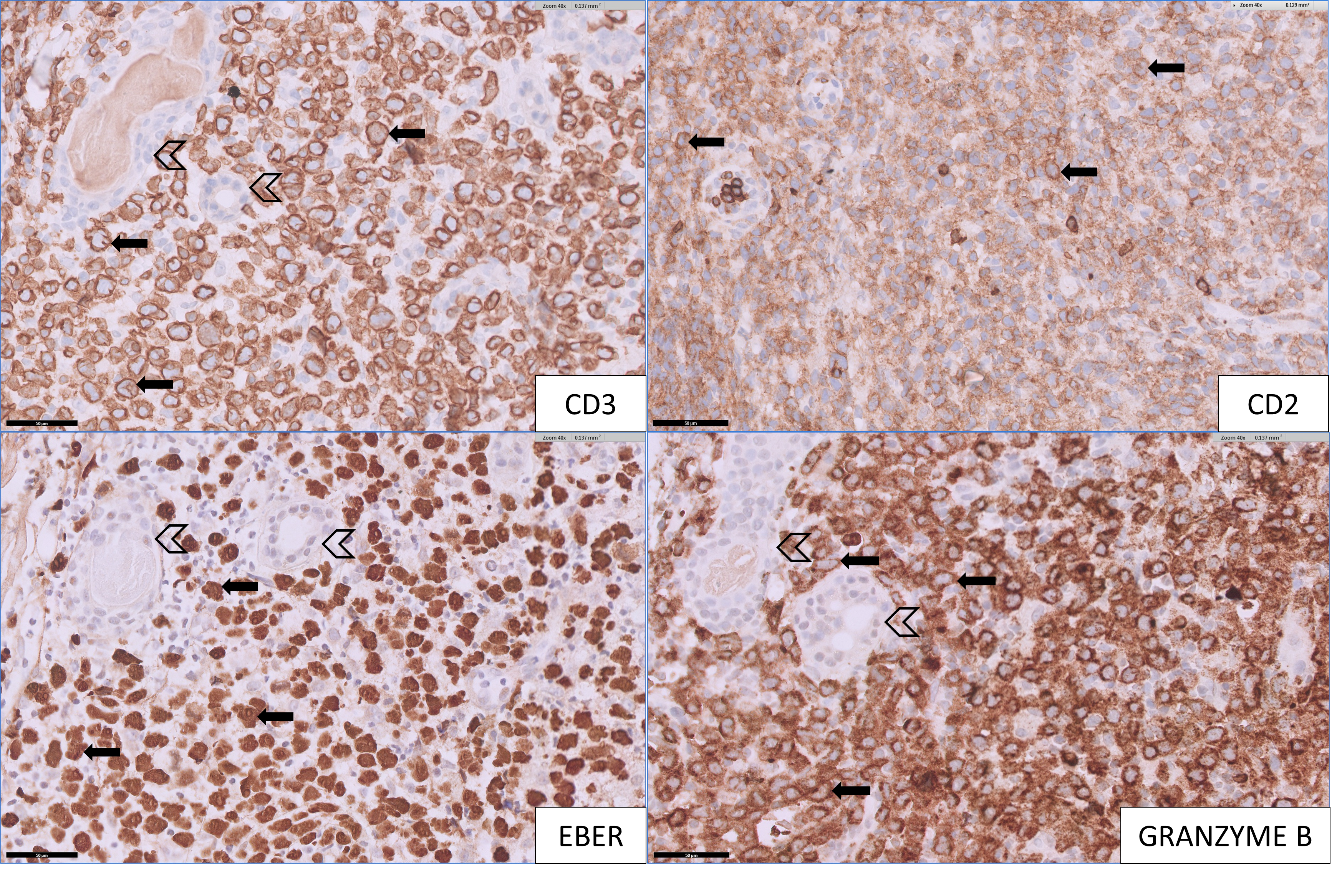
Figure 2: [Top left] Medium to large lymphomatous cells show cytoplasmic staining for CD3 (arrow), adjacent to remnant sweat glands (open arrow head). [Top right] Medium to large lymphomatous cells show weak to moderate positivity for CD2 (arrow), which is diminished compared to the few strongly staining scattered reactive small T-cells. [Bottom left] Lymphomatous cells show diffuse positivity for EBER (arrow), adjacent to remnant sweat glands (open arrow head). [Bottom right] Lymphomatous cells show positivity for granzyme B (arrow), adjacent to remnant sweat glands (open arrow head).
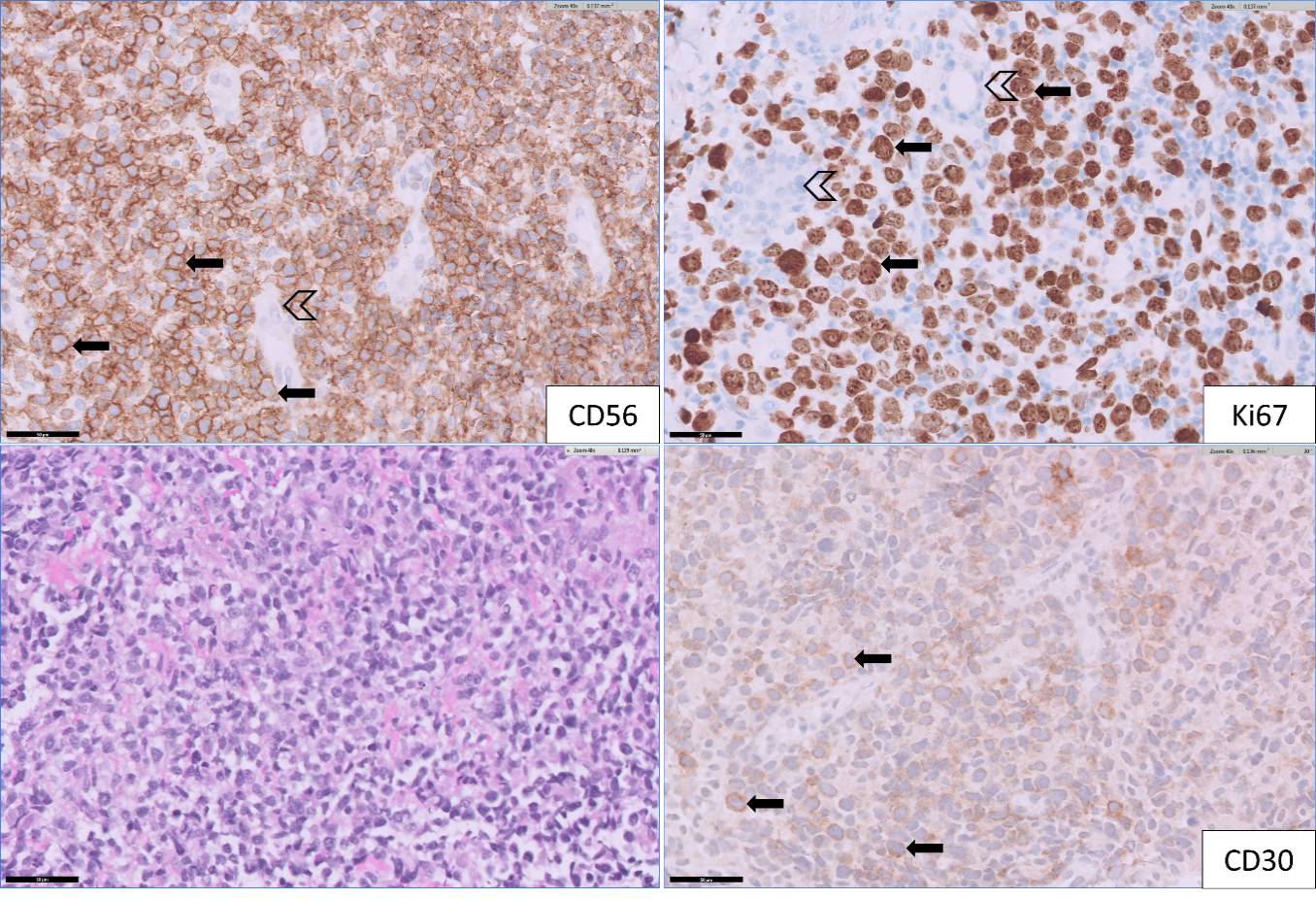
Figure 3: [Top left] Medium to large lymphomatous cells (from thigh specimen) show diffuse positivity for CD56 (arrow), admixed with some remnant small vessels (open arrow head). [Top right] Lymphomatous cells are highly proliferative as highlighted by Ki67 (arrow). Adjacent remnant sweat ducts are negative for Ki67 (open arrow head). [Bottom left] H&E stain (400x magnification): This shows medium-to-large cell lymphomatous infiltrate. [Bottom right] Immunohistochemistry CD30 (400x magnification) from arm specimen: Medium to large lymphomatous cells show weak positivity for CD30 (arrow) in minor subset of lesional cells.
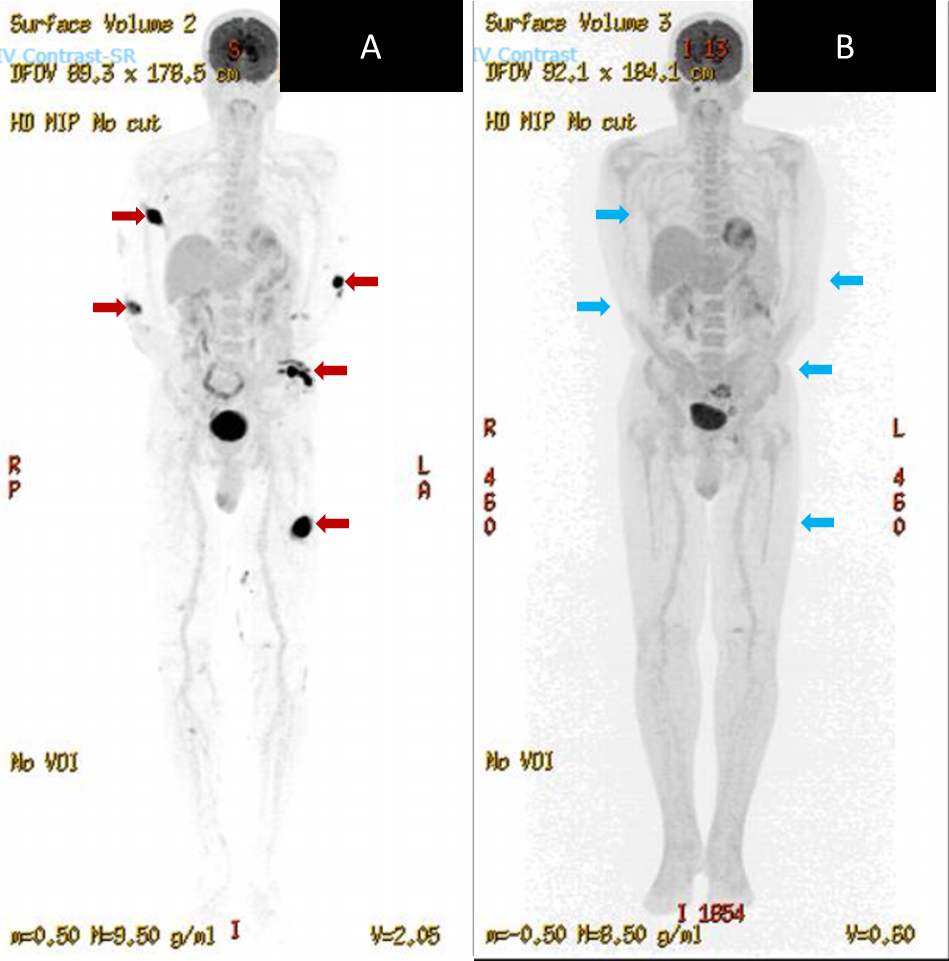
Figure 4: [Left] PET-CT at diagnosis showing multiple enhancing and hypermetabolic skin nodules (red arrows). [Right] PET-CT done 5 months following initiation of treatment showing resolution of cutaneous nodules (blue arrows indicating sites of previous tumours)
Discussion
Extranodal natural killer/T-cell lymphoma (ENKTL) is deadly with an aggressive course. Primary cutaneous ENKTL describes disease that initially manifests in the skin. L-asparaginase-based chemotherapy with high-dose radiation therapy forms the basis of treatment in early disease and may be curative1. However, prognosis in advanced disease is invariably poor and developments in treatment have been impeded by the rarity of this disease. Asparaginase-based chemotherapy followed by consolidation with autologous transplantation may be used in advanced stages but treatment outcomes remain extremely poor1.
BV is a CD30-targeted antibody-drug conjugate that delivers vedotin (monomethyl auristatin E), a cytotoxic agent, to CD30-expressing cells2. CD30 has been identified as a therapeutic target due to its relative overexpression in specific tumour types and limited expression on normal cells3. CD30 expression is commonly associated with Hodgkin’s lymphoma and anaplastic large cell lymphoma, but may also be seen in certain T- and B-cell lymphomas4. Clinical trials with BV have shown impressive results in the treatment of relapsed/refractory Hodgkin’s lymphoma and T-cell lymphomas – in particular, cutaneous T-cell lymphoma (CTCL), systemic anaplastic large-cell lymphoma and other peripheral T-cell lymphoma (PTCL) subtypes3.
ENKTL typically exhibits CD2, CD56, and cytoplasmic CD3 positivity5. CD30 expression was demonstrated in 36.4% of patients with ENKTL in a study by Hong et al6. Theoretically, BV could be effective in the treatment of CD30-positive ENKTL. However, there are no clinical trials evaluating the efficacy of BV in ENKTL7. To date, there have been only 2 case reports where the use of BV therapy led to complete response. Both patients had relapsed/refractory ENKTL4,8.
In theory, the level of CD30 expression should influence the efficacy of BV, considering that CD30 is the molecular target of the drug. Interestingly, CD30 expression in our patient showed only weak positivity, with levels ranging from 5-20% from both specimens. A recent study by Jagadeesh et al in 2022 - which examined the response to BV by CD30 expression in Non-Hodgkin Lymphoma - showed that response to BV was observed in patients with all levels of CD30 expression9. The same study found no significant differences in overall response between patients with CD30 expression ≥10% or <10%9. The authors concluded that CD30 expression does not predict clinical response to BV and elucidating a meaningful threshold level of CD30 expression remains challenging. These findings were also demonstrated by Jacobsen et al in 2015, where the study found no statistical correlation between response to BV therapy and level of CD30 expression in patients with relapsed/ refractory diffuse large B-cell lymphoma10.
A number of hypotheses have been proposed to account for observable clinical response to BV independent of the degree of CD30 expression. These include an intra- and inter-tumoral heterogeneity of CD30 expression, the dynamic nature of CD30 expression on tumour cells and alternative modes of action of BV; such as through direct cytotoxicity thought to be enhanced by the bystander effect, immunogenic cell death and antibody-dependent cellular phagocytosis9.
We describe complete response using BV upfront in addition to conventional chemotherapy in our patient with primary cutaneous ENKTL-NT. Repeat PET-CT 5 months following diagnosis demonstrated resolution of all cutaneous nodules (Figure 4). At the time of writing (2 months after his interval PET-CT), our patient remains in remission. There are few reports of successful treatment of primary cutaneous ENKTL with BV upfront, as opposed to salvage therapy.
Conclusion
We add to the growing body of evidence of successful use of BV in CD30-positive ENKTL, with emphasis on primary cutaneous disease. Upfront BV could be efficacious in CD30-positive ENKTL.
References
- Allen PB, Lechowicz MJ. Management of NK/T-Cell Lymphoma, Nasal Type. J Oncol Pract. 2019; 15(10): 513-520. doi: 10.1200/JOP.18.00719.
- Scarisbrick JJ. Brentuximab vedotin therapy for CD30-positive cutaneous T-cell lymphoma: a targeted approach to management. Future Oncol. 2017; 13(27):2405-2411. doi: 10.2217/fon-2017-0263.
- Van Der Weyden C, Dickinson M, Whisstock J, et al. Brentuximab vedotin in T-cell lymphoma. Expert Rev Hematol. 2019; 12(1): 5-19. doi: 10.1080/17474086.2019.1558399.
- Kim HK, Moon SM, Moon JH, et al. Complete remission in CD30-positive refractory extranodal NK/T-cell lymphoma with brentuximab vedotin. Blood Res. 2015; 50(4): 254-256. doi: 10.5045/br.2015.50.4.254.
- Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005; 19(12): 2186-2194. doi: 10.1038/sj.leu.2403955.
- Hong J, Park S, Baek HL, et al. Tumor cell nuclear diameter and CD30 expression as potential prognostic parameter in patients with extranodal NK/T-cell lymphoma, nasal type. Int J Clin Exp Pathol. 2012; 5(9): 939-947.
- Hu B, Oki Y. Novel Immunotherapy Options for Extranodal NK/T-Cell Lymphoma. Front Oncol. 2018; 8: 139. doi: 10.3389/fonc.2018.00139.
- Poon LM, Kwong YL. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann Hematol. 2016; 95(5): 847-849. doi: 10.1007/s00277-016-2627-9.
- Jagadeesh D, Horwitz S, Bartlett NL, et al. Response to Brentuximab Vedotin by CD30 Expression in Non-Hodgkin Lymphoma. The Oncologist. Published online August 10, 2022: oyac137. doi: 10.1093/oncolo/oyac137.
- Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015; 125(9): 1394-1402. doi: 10.1182/blood-2014-09-598763.
