Silica and Connective Tissue Disorders: The Important Role of the Dermatologist
Deborah H Yates1,2,3*, Susan E Miles4,5
1Department of Respiratory Medicine, St Vincent’s Public Hospital
2St Vincents Clinical School, UNSW
3Holdsworth House Medical Practice, Sydney, NSW
4Department of General Medicine at Calvary Mater Newcastle
5University of Newcastle, Newcastle, NSW, Australia 2308
Abstract
Dermatological manifestations of connective tissue diseases (CTDs) are common and frequently precede other symptoms. Thus, dermatologists may be the first clinicians to diagnose these disorders. Silica exposure is an acknowledged cause of several CTDs, but this is under-appreciated by clinicians, who may also be unaware of the wide range of jobs in which silica exposure can occur. The CTDs associated with silica exposure include systemic sclerosis (SSc), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), anti-neutrophil cytoplasmic antibody (ANCA) positive vasculitis and overlap syndromes. Silica-related systemic sclerosis (Si-SSc) is associated with a specific antibody profile and more severe disease. Silicosis has re-emerged worldwide recently due to several new workplace exposures, including a new type of silicosis (artificial stone (AS) silicosis), which is associated with a particularly high rate of auto-antibody formation. Dangerous work practices are still occurring. This article summarises recent literature on the topic of the resurgence of silicosis and silica-induced CTDs and reminds dermatologists of the importance of taking a thorough occupational history in all patients. Early intervention in CTDs and reduction in dust exposure can reduce risk and improve prognosis. Treatment options are rapidly improving.
Introduction
Autoimmune diseases (AID) are complex disorders involving immune responses to self-antigens. Although of unknown etiology, these are currently believed to result from interactions between genetic and environmental factors1. Connective tissue disorders (CTDs) are a subset of AID, affecting tissues such as the skin, joints and cartilage. Skin manifestations occur in almost all CTDs, making the dermatologist a key player in the diagnosis and management of these clinically heterogeneous conditions. Cutaneous manifestations may occur before systemic disease and can enable early risk stratification into subtypes, which affect prognosis. Both local and systemic diseases are increasingly treatable using modern therapeutic approaches2.
There are many different CTDs, and evidence about their origins increasingly suggests an environmental contribution toward their development; collectively these are frequently encountered in clinical practice. Estimates of CTD prevalence in developed countries range between 3-7% of the population3, and they are generally more common in women. There is now convincing evidence that several CTDs, including scleroderma (SSc), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) are associated with exposure to crystalline free silica (CFS), and evidence regarding other CTDs is evolving. These are not new findings, the relationship having been suggested over a century ago4.
Interest in this area has been stimulated by the recent re-emergence of severe silicosis in young workers, with several deaths from this totally preventable disease. The tragedy of denim-associated silicosis in the early 2000s in Turkey showed that a fashion item could literally become something “to die for”5. These workers were sandblasting, a practice which has been known to be highly dangerous for centuries. Also, a severe new type of progressive silicosis was described in 20106, now known as artificial stone (or engineered stone) silicosis. Existing legislation nominally restricted silica exposure to safe levels Nonetheless it was late-stage disease which first presented to clinicians7, highlighting the gap between regulation and actual workplace practice. Engineered stone (or artificial stone, AS) silicosis has subsequently been found in many other countries including Israel, Italy, China, Belgium, the USA and Australia8-12. Case finding studies in Australia have demonstrated that this is disturbingly common even in a wealthy, highly regulated country13.
AS dust exposure is associated with a particularly high rate of autoimmunity9,13. Because of the long latency between exposure and disease, the future legacy of these exposures has yet to be revealed. At the same time, there has also been a resurgence of cases of silicosis in the coal mines of the USA, related primarily to higher silica exposures14, and the association between coal dust exposure and arthritis has been re-visited15. These developments have been described at a time when basic knowledge regarding the pathophysiology of adverse effects of silica exposure is improving, revealing that low level silica exposure is probably more dangerous than has previously been believed16-18.
These events have highlighted the relevance of inhaled exposures in the pathogenesis of CTDs and the need for clinician awareness. Clinicians cannot assume that modern workplace dust exposures are too low to produce significant disease. We need to take a continued interest in the work that our patients do and remember to take a careful history of potential environmental exposures, some of which may have occurred many years ago. Clinicians still play a vital role in identifying and understanding these disorders.
Silicosis: a disease revisited
Silicosis is a disease most dermatologists will probably remember from their medical school days, but hopefully few will encounter in their day-to-day practice. Silica and silicates are ubiquitous minerals which are the basic components of soil, sand and granite. Silicosis is a fibrotic lung disease which only occurs with relatively high silica inhalation and has specific radiological and pathological characteristics12. Silicosis is mainly caused by the inhalation of free silica (silicon dioxide (SiO2)), a crystalline form of the element silica. Respirable crystalline silica (RCS) is that fraction of SiO2 which can be inhaled into the peripheral lung and comprises the “silica” which is regulated and measured in workplaces. RCS is usually found as quartz, which is the major constituent of most soils and rocks. Thus, it is frequently encountered in mining, quarrying, road building and is used in a surprisingly wide variety of industrial processes. Silicosis can also occur after inhalation of amorphous silicates, such as China clay and diatomaceous earth18. Silicates are SiO2 linked with another element, usually a metal oxide, and are also very common in the occupational environment.
Silicosis differs from silica exposure, where no pulmonary fibrosis is present, but where a pathophysiological response to silica exists. The dose required for production of disease is not uniform among humans and no threshold for the fibrotic response has been documented19-21. Most silica-related disorders (including CTDs) have been described at levels which are too low to produce pulmonary fibrosis, and the actual dose which is safe for inhalation is debatable21,22. Genetic susceptibility and co-exposure to other environmental factors are also very likely to play a role. Nonetheless, it is clear that the more RCS inhaled, the greater the risk of disease. Freshly fractured particulate silica is more toxic than older silica particles12,21,22, and thus the risks from cutting, grinding and blasting are higher than simply working with weathered rock. Silicone (as used in breast implants), is a totally synthetic product, and does not cause silicosis, although it has been implicated in autoimmune disorders24.
It has taken many years for the full range of effects of silica dust inhalation to be appreciated. As well as silicosis and CTDs, RCS is now known to cause several other lung disorders, including lung cancer, diffuse interstitial pulmonary fibrosis (or diffuse dust-related fibrosis), chronic obstructive pulmonary disease (COPD, which includes emphysema), and is also implicated in renal disease12,23,25. The development of all these diseases is dose-related. In real life, patients are exposed to a mixture of different dusts, and often also other inhaled substances such as tobacco fumes, vapours and organic dusts, especially over a long working career. Thus, it can be difficult to tease out the different effects of exposures, which is why it is very helpful to have exposures documented contemporaneously.
Prevention of silica-related disease
Silica-related disease is believed to be totally preventable using modern dust control measures ie reduction of dust production, dust suppression (often using water), and ventilation. The use of masks (or personal protective equipment, PPE), represents a last-ditch control method which should not be relied upon20. Early diagnosis (or early documentation of excessive exposure) with reduction or removal from exposure will prevent or slow progression and enable treatment if required. This is the rationale for the periodic surveillance programs which are mandatory in many workplaces26,27, but which may not actually occur in practice.
The wide range of occupations in which silica exposure may occur:
Silica is the most abundant mineral on earth, and clinicians may not appreciate just how many jobs involve exposure to silica and silicates (Table 1). Silica is invisible to the naked eye, so exposure may go unnoticed. Freshly fractured particulate silica (crystalline silica or quartz) is produced in any job which involves drilling or fracturing rock25. Silicosis has traditionally been recognized in the so-called dusty trades (mining, particularly coal, gold, shale and granite), polishing (e.g., the knife grinders of Sheffield) and in tunnelling. Exposure commonly occurs nowadays in construction of dams and roads, and in the pottery, ceramics, brick, tile and cement industries20,28,29. Silica is widely used in many industrial processes. More recently, silicosis has been described in less obvious trades like jewellery29 and dentistry31. It is estimated that more than 40 million workers are at risk from exposure from RCS32-34. These are usually (but not always) manual workers, from lower socio-economic groups.
Table 1: Jobs commonly involving exposure to crystalline free silica (CFS).
|
Stone and brick masonry: paving, surfacing, angle grinding |
|
Artificial stone fabrication and installation: manufacture, cutting, drilling, polishing |
|
Sandblasting: cleaning and priming of surfaces, glass etching, stone washed denim |
|
Concreting: air polishing, jackhammering, chiselling |
|
Construction: plastering, roofing, rendering |
|
Demolition: labouring, plant operating, cleaning |
|
Mining: cutting, blasting, tunnelling, bolting |
|
Quarrying: excavation, earth moving, stone processing |
|
Tunnel construction: drilling, boring |
|
Hydraulic fracking: gas and oil wells |
|
Road construction and maintenance: earthworks, asphalt, concrete and bitumen laying |
|
Foundry work: metal casting, surface cleaning |
|
Pottery work: porcelain, ceramics, clay |
|
Jewellery production: grinding, polishing, sanding |
|
Glass manufacture: handling, mixing and transporting raw materials, sandblasting |
|
Dental technicians: levelling, smoothing and polishing of porcelain prostheses |
|
Agriculture: inorganic dust exposure in the stockyard, ploughing and harvesting |
Several work practices are particularly associated with a high risk of silicosis, such as abrasive blasting using sand (sandblasting)35 and cutting or polishing artificial stone. Sandblasting involves forcibly propelling a stream of abrasive material against a surface under high pressure – a system ideally suited to produce respirable particles, which may derive not only from the abrasive material but also from the surface blasted. Sandblasting was the practice causing the Turkish denim jean outbreak of silicosis in 20065 and was subsequently banned due to popular outcry36. Substances other than sand can be substituted e.g., metals, steel grit, coal slag, glass beads, or “softer” ones like crushed nut shells or magnesium sulphate, in which case the lung disease may look atypical. Abrasive blasting is still common in industries involving ship, car and pipe repair and production of monuments or signs.
The introduction of new products can also change risks. Engineered (or artificial) stone is a relatively new building product made by mixing finely crushed rock with polymeric resin. It is available in hundreds of different varieties and is very attractive; the product is cheaper and more durable than traditional stones and sales have increased exponentially over the last 15 years. The content of RCS in AS can be very high (approximately 90 % compared to 3% in natural marble and 30% in granite). AS is factory manufactured then cut to size on site using power tools, producing very high levels of RCS25; high levels of silica nanoparticles are generated37. Despite excellent knowledge regarding the hazards of use of high silica content-stones and existing regulations requiring dust control measures, control measures have been rarely used in practice13. Even dust suppression by using water (wet cutting), a practice which has been known for centuries to significantly suppress silica dust levels, has been neglected, as has been PPE. The silicosis which resulted in these workers has been more rapidly progressive than with other types of silicosis and has been accompanied by a high incidence of positive autoimmune autoantibodies, up to 60% in some studies9,13. Lung transplants have been required in several patients and the full spectrum of autoimmune disease which will evolve from this exposure is as yet unknown. Silica nanoparticles may play an important role37,38, acting via impairment of macrophage efferocytosis39.
New practices such as hydraulic fracking are also likely to involve significant RCS exposure. Fracking to recover hydrocarbons involves using large volumes of water along with a solid (called a proppant) and sand is often used for this purpose. The US National Institute of Occupational Safety and Health (NIOSH) has recently recommended substitution of sand by non-silica proppants to reduce dust levels, after a study showed that more than 50% of measured exposures in several US states exceeded the permissible exposure limits40.
Unrecognised silica exposure and that occurring in non-occupational settings
Significant RCS exposure also may occur in non-occupational settings, but this has been much less studied. Non-occupational exposures can occur in ordinary life, for example when using scouring powders, cleaning dusty clothes, and in do-it-yourself hobbies41. Silicosis can occur from inhalation of agricultural and desert dusts, particularly dust storms42. Silicosis has also been described in animals including horses and camels43,44. These types of activities tend to pass unnoticed and respiratory protection is seldom used; questions about these exposures are hardly ever recorded in questionnaires or taken into account in case-control or cohort studies.
Silica and connective tissue disease
There is a now a convincing body of evidence confirming an association between RCS exposure and CTDs1,45-48. The evidence for a causative relationship is currently most convincing for SSc and RA. Pre-clinical features of autoimmunity, such as autoantibodies, as well as clinical autoimmune diseases occur in the absence of established silicosis, suggesting that events prior to fibrosis are important for silica-induced autoimmunity. Basic science and animal studies have elucidated potential mechanisms16,17,38. These suggest that the ingestion of silica particles by alveolar macrophages activates the innate immune system leading to the production of proinflammatory cytokines and pulmonary inflammation (Figure 1). This leads to activation of adaptive immunity, loss of tolerance and the production of autoantibodies. Fibroblasts are stimulated to proliferate and produce collagen which encases silica particles resulting in fibrosis and silicotic nodules16. There are several excellent reviews and meta-analyses available for the interested reader46,47 as well as comprehensive summaries from government agencies48-50.
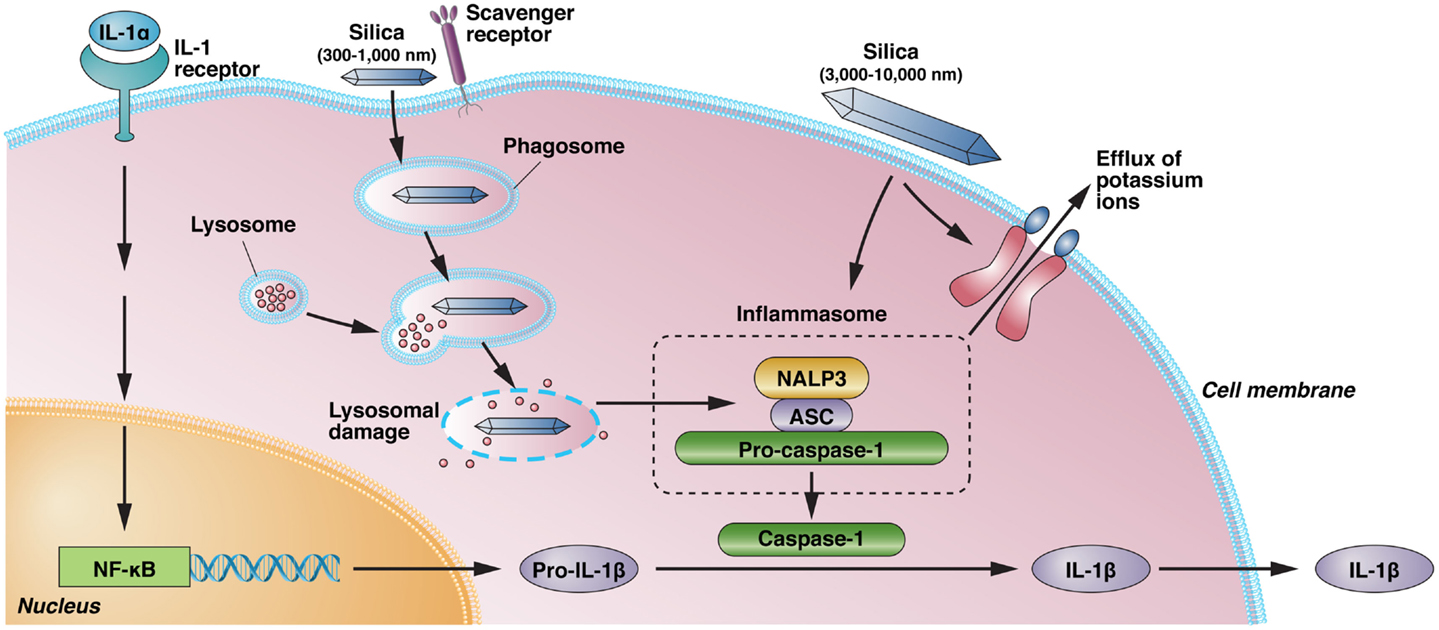
Figure 1. Silica-induced activation of inflammasome and IL-1 production. IL-1α, released from alveolar macrophages following crystalline exposure, results in NF-κB activation and transcription and translation of pro-IL-1β. Phagocytosis of crystalline silica leads to phagosomal damage and release of phagosome contents into the cytoplasm. This results in the activation of NALP3 and its association with the intracellular adapter protein ASC, which combines with and activates pro-caspase-1. The resulting inflammasome cleaves pro-IL-1β to the proinflammatory IL-1β. However, binding of immobilized silica crystals to the cell membrane of macrophages is also sufficient to induce IL-1β without evidence of lysosomal damage. Activation of the NALP3 inflammasome by silica also results in efflux of intracellular potassium ions, suggesting a possible interaction of silica with a membrane-associated protein, but it is unclear if K+ efflux following binding of immobilized silica crystals to the cell membrane results in inflammasome activation. Scavenger receptors have a role in the recognition and uptake of silica. NALP3, NACHT, LRR, and PYD domains-containing protein 3; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; NF-κB, nuclear factor-κB; IL, interleukin.
*Reproduced with kind permission from ref 16.
Systemic sclerosis (SSc)
Systemic sclerosis is a rare disease with a widely varying incidence and a female predominance. The incidence of SSc in women reported to be up to 14 times higher than that in men51. The low concordance rate reported in twin studies and the unequal geographic distribution of SSc51-53 suggest that environmental factors are relevant to disease initiation. Thus, when SSc occurs in a man, an occupational or environmental contribution should be suspected.
Knowledge about the link between silica and and SSc is not new. This was first described over 100 years ago by the Scottish physician Bryrom Bramwell54, who specifically mentioned the importance of dermatologists in diagnosing scleroderma. In his paper, Bramwell notes that “cases of diffuse sclerodermia come at least a frequently under the care of the general physician as under the care of the dermatologist”, and that he was able to tell the occupation of the patient “as soon as I saw and felt the hands”. It was not until 1957 that Erasmus from South Africa performed another study in miners, attempting to compare the incidence of SSc in gold miners with that in the general population55. Erasmus described 17 cases of SSc in miners who had “gross” sclerodermatous skin changes and had worked underground in gold mining for an average of 9 years. Only 6 had radiological silicosis, and there was an average 18 years from first dust exposure to when skin changes had started. The sites and types of skin lesions are carefully documented in his report. All had “gross changes in the skin of the fingers, which were thickened, taut and glistening”, and 12 of the 17 had Raynaud’s phenomenon despite the warm South African climate. Erasmus’ report stimulated several more case series and case-control studies in later years56-58.
In the 1960s Gerald Rodnan, a rheumatologist at the University of Pittsburgh, USA, became interested in the association and again confirmed “heavy exposure to silaceous dusts” in the men he studied. His patients were mainly coal miners although some worked as enamellers, pottery and foundry workers59, and again Raynaud’s phenomenon was common (10/26). Rodnan later developed the Rodnan skin score, a validated outcome measure for skin thickness in SSc60 which is still used today.
Since then, many case-control and cohort studies been conducted61-64 and summarised in reviews and meta-analyses64-68. In general, these confirm a significant association between silica exposure and SSc in males, more marked with cumulative exposure64-66. The association between SSc and silica appears to be with more severe forms of the disease69. A consensus report from National Institute of Environmental Health in 201248 listed silica as an environmental exposure that “we are confident contributes to the development of human autoimmune disease”. This report summarised the evidence from 16 studies (3 cohort, 9 case control and 3 mortality studies). As would be expected, the different studies had a range of relative risk (RR) estimates, but 11 of the 16 RR estimates were >1.5. The mean RR from all studies was 3.2 (1.89-5.43), without a raised RR in females (1.03; 0.74-1.44). Cohort studies showed a RR of 15.49 (4.54-52.87), possibly due to higher exposures, while case control studies showed a lower RR of 2.24 (1.65-3.31)47 (Table 2).
Table 2: Meta-analysis of occupational silica exposure as a risk factor for scleroderma
|
Author |
Study years |
Study type |
Effect size (95% CI) |
|
Rubio-Rivas 2017 (68) |
1960-2014 |
15 case control 4 cohort
|
overall OR= 2.81 (95%CI 1.86–4.23; p < 0.001) overall RR= 17.52 (95%CI 5.98–51.37; p < 0.001) |
|
McCormic 2010 (65) |
1949-2009 |
9 case control 3 cohort 4 other |
Combined estimator of relative risk (CERR) 2.24 (95% CI, 1.65–3.31) CERR 15.49 (95% CI, 4.54–52.87) |
These data have been confirmed in many countries throughout the world, including in a recent 2020 Australian study where more than 30% of men in a systemic sclerosis registry of 1670 patients reported a history of silica dust inhalation compared with 3.7% of women70. The relationship between silica exposure and scleroderma is now so convincing that an accompanying editorial pointed out that this “could no longer be ignored”71. This has also been acknowledged by several international agencies including the UK Industrial Injuries Advisory Council IIAC49) and the French Agency for Food, Environmental and Occupational Health and Safety (ANSES)50. The latter concluded that the relationship is “certain and strong”.
Clinical and autoantibody features
Although the clinical, serological and immunological features of Si-SSc were initially reported as identical to those of idiopathic disease2,58,60-62, recent studies have suggested otherwise. Improvements in antibody testing, new methods of clinical assessment, and better clinical registry data have allowed better discernment between groups. Si-SSc is associated with more severe, progressive disease, with Raynaud’s phenomenon, and progressive fibrosing interstitial lung disease (PF-ILD)63-68.
Clinical studies contrasting silica-exposed and non-exposed patients have been reported61,63-65,68, and there have been several meta-analyses65,68, with a systematic review from 2015 noting 32 published studies and clinical data on 254 patients, the vast majority of whom were men (96%)67. Diffuse SSc predominated, with an overall prevalence of interstitial lung disease of 81%, and lower overall survival compared with those unexposed to silica.
More recent data has confirmed that silica exposure is highly associated with systemic disease and there are several clinical features which can be easily clinically assessed and are predictive of survival63-69. Raynaud’s phenomenon (RP) affects almost all patients (>95%) and is one of the three criteria for early diagnosis of scleroderma, along with puffy fingers and ANA positivity71, but these features do not predict prognosis. Nail fold capillaroscopy (NFC) can assess severity of microvascular damage and is considered a biological marker of disease progression, and predictive of multi-organ involvement in SSc72. Digital ulcers also predict survival and are associated with pulmonary hypertension72. Diffuse disease, interstitial lung disease, digital ulcers, myocardial dysfunction and positive anti-Scl-70 are more closely associated with Si-SSc and indicate a worse prognosis63,65,70.
These clinical features have been confirmed recently by Patel et al in the Australian SSc cohort70, where clinical and immunological features of silica related and unrelated SSc were compared. In the silica-exposed group, there was a higher frequency of diffuse disease subtype, anti-Scl 70 antibody positivity, joint contractures and higher modified Rodnan skin score. Although ILD was more common in the silica-exposed group, the difference in prevalence between those exposed and non-exposed did not reach statistical significance (32.5% vs 27.0%, p=NS). All physician and patient-reported outcomes were worse in SSc male patients exposed to silica compared to those unexposed (p=0.02). Thus, simply asking the patient about work exposure to silica predicted worse physical function and higher disease activity.
Autoantibody testing is helpful for diagnosing SSc and also for distinguishing between different disease subtypes73. Autoantibodies are detected in > 90% of patients with SSc, usually anti-nuclear antibodies (ANAs), which are positive at a titre of >1:160. Two ANAs are relatively specific: anti-topoisomerase-1 (otherwise known as anti-Scl-70), and an anti-centromere antibody (ACA) called RNA polymerase III (RNApol3). Other anti-centromere antibodies (ACAs) are less specific for SSc, and more likely to be found in the limited cutaneous subset of SSc or CREST syndrome. ACAs are also produced in SLE, Sjögren's syndrome, RA, and primary biliary cholangitis, thus identifying SSc overlap syndromes71-75.
Anti-topoisomerase antibodies (anti-Scl 70 or ATAs) and anti-RNA polymerase antibodies (ARAs) are highly specific for SSc and rarely detected in other autoimmune diseases76. They can be of different immunoglobulin subtypes eg IgG, IgM or IgA. ATAs are up to 99.6% specific although significantly less sensitive (24%) for SSc. ATAs are associated with diffuse SSc and a higher risk of interstitial lung disease (ILD). However, ATAs can also be positive in some cases of limited SSc. A particular ARA called RNA pol 3 or anti-RNA polymerase III is a marker of rapidly progressive skin involvement and an increased risk of renal crisis. ILD is linked to anti-topoisomerase, anti-U11/U12 RNP and anti-Th/To. Also, RNApol3 is topical because its emergence has been shown to coincide with the development of malignancy, suggesting that some SSc can be initiated by autoantigen mutation within the patient’s cancer77 Interestingly, in patients with positive RNApol3 the risk of different cancer types differs according to skin subtype. Thus, patients with SSc had an increased breast cancer risk (SIR 5.14, 95%CI 2.66–8.98), while those with limited scleroderma had a high lung cancer risk (SIR 10.4, 95%CI 1.26–37.7). In contrast, patients with ACAs had a lower risk of cancer (SIR 0.59, 95% CI 0.44–0.76)76. Thus, skin subtype combined with autoantibody subtype can be highly relevant to clinical course.
It seems likely that the observed variation in incidence of positive auto-antibodies (between 10% - 60 %) in patients with Si-SSc could reflect the wide range of different doses of silica acting on different genetic susceptibilities9,76; more information is needed on this topic. Also, brief exposure to dust with a high silica content could be associated with SSc, but this is seldom recorded. There is a need for further inquiry in this area using a multidisciplinary generalised approach. Hopefully, this will be assisted by a new initiative for SSc which is currently emerging using the 2013 ACR/EULAR (American College of Rheumatology/European League Against Rheumatism) classification which aims to select patients very early in the disease process78. This (the Very Early Diagnosis Of SSc or VEDOSS approach) employs NFC and clinical detection of “puffy fingers” as well as ANA testing78. Dermatologists would be key contributors to this endeavour79.
Overall, thus, it is clear that where dermatological and autoantibody features of SSc occur in a man, an additional history of silica exposure can identify a group of patients who are likely to progress and develop complications, and who should probably be followed up closely and treated earlier.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is another CTD with a female predominance. Although it has well described genetic predisposing factors (particularly HLA-DRB1 (the “shared epitope”), heritability only accounts for ~40–50% of seropositive RA, and ~20–30% of seronegative RA80. RA has been linked to exposures to many environmental agents, particularly in men81, and there is now a substantial evidence base regarding RA and silica exposure.
The identification of anti-citrullinated protein antibodies (ACPAs, or anti-CCPs) in 1998 enabled better elucidation of potential mechanisms in the pathogenesis of RA, including the role of environmental triggers. ACPAs are present in the majority of patients with RA and are highly specific (88-96%), allowing early diagnosis even before clinical manifestations occur82. Cigarette smoking is an important environmental trigger for RA and is, like silica, associated with ACPA positive disease. The lung is probably an initiating site of injury83, in bronchus-associated lymphoid tissue (BALT), with an interaction between two or more toxins potentially enabling auto-antibody formation. Although other environmental risk factors have also been described (e.g., mineral oils, farming and pesticide exposure, electrical work, pollution), the strongest association documented to date with occupational agents is with silica.
The association between RA and silica dust exposure was first reported by clinicians many years ago by both Colinet in Belgium84 and Anthony Caplan in Wales85. Caplan described multiple, rounded opacities on the chest X rays of coal miners which were not pneumoconiosis. Miners either had RA or those later developed this; he noted these were associated with rheumatoid nodules of the skin. He suggested that “in the majority of cases the association of the two conditions is more than coincidental”. This association was confirmed in later studies and much epidemiological research86-94.
Collectively, this work has consistently reported a raised RA in individuals exposed to silica (Table 3)91,92. A recent meta-analysis92 found a significant association between occupational exposure to silica with a relative risk of developing RA of 2.59 (95% CI 1.73-3.45), similar to the increased risk produced by smoking (2.49, 95% CI 1.13-3.86). In general, the risk is for ACPA-positive RA, consistent with Caplan’s original description. An intriguing interaction with cigarette smoking has also been noted. In one study, the risk for ACPA-positive RA among silica-exposed current smokers was 7.4 times higher than among non-smokers without silica exposure, exceeding the risk expected from the separate effects of silica and smoking89. However, the recent Swedish National Registry Case-Control Study, published in 2021, found a statistically significant increase in OR for both seropositive and seronegative RA but in men alone. Relative risks were much lower at 1.22 for seropositive RA and 1.23 for seronegative RA94.
Table 3: Systematic Review and Meta-analysis on the Association Between Occupational Exposure to Crystalline Silica and the Risk of Developing Rheumatoid Arthritis
|
Author |
Study year |
Study design |
Effect Size (95%CI) |
||
|
Morotti 2021 (91) |
1986- 2019 |
7 case control 5 cohort |
OR =1.94 (95% CI 1.46–2.58). |
||
|
Mehri 2020 (92) |
1987-2018 |
8 case control 5 cohort 2 cross sectional |
OR = 2.59 (95% CI = 1.73 - 3.45). |
||
These studies suggest a causative association between ACPA-positive RA and silica exposure. There is currently insufficient information regarding any particular clinical features which could distinguish between silica-induced and other types of RA: this needs to be examined in the future.
Systemic Lupus Erythematosus, ANCA positive vasculitis, Sjogren’s syndrome and overlap syndromes
There is less evidence available on exposure to silica and SLE, anti-neutrophil cytoplasmic autoantibody (ANCA) positive vasculitis, Sjogren’s syndrome and overlap syndromes. Because of their rarity, these diseases are difficult to study, and the ANCA-positive vasculitides comprise a number of different diseases which have been reclassified since their initial description. These include granulomatosis with polyangiitis (formerly known as Wegener’s granulomatosis), microscopic polyangiitis and eosinophilic granulomatosis with polyangiitis (formerly called Churg-Strauss syndrome)95. If silica does induce vasculitis, then this could underlie the observed association between renal disease and silica exposure.
Silica exposure has been implicated in the pathogenesis of SLE for years in clinical reports and case-control studies95-98. Cases of SLE have been repeatedly identified among workers heavily exposed to silica95,97-98. Case control studies from the Americas and Europe support an association between silica exposure and SLE, but not all have shown a risk of biopsy-confirmed SLE nephritis96,98.
Several case-control studies from Europe and the USA support the association between crystalline silica exposure and increased risk of anti-neutrophil cytoplasmic antibody (ANCA)-related diseases, including ANCA positivity, ANCA-positive small vessel vasculitis97-101. The RR associated with silica exposure was greater than 2.0 compared with non-exposed individuals in almost all studies96-98,100,101, and a dose effect was reported. Nonetheless, a recent large case-control study from Sweden did not find a significant association of Wegener’s granulomatosis with 32 occupations evaluated99. Another more recent nationwide study from Denmark demonstrated an increased risk of SSc, RA, SLE and small vessel vasculitis in men but less in women102. The relative risk was lower for SLE and vasculitis than for SSc and RA but suggestive of a causative effect.
Overall, the results of these studies are inconsistent. The literature has been relatively recently summarized both by the French Agency for Food, Environmental and Occupational Health & Safety (ANSES)50. and in two reports from the UK Industrial Injuries Advisory Council49,97. These concluded that there was a possible, but not certain, association between silica exposure and ANCA-associated vasculitis. The situation with regard to Sjogren’s and overlap syndrome is similarly difficult to confirm with the current evidence base but seems plausible.
Conclusions
Despite silica being one of the best known of occupational exposures, this is still causing significant disease in the 21st century. Knowledge of the spectrum of silica’s pathogenic effects has broadened with improved scientific understanding in the 20th and 21st centuries, and silica-related disorders are now acknowledged to be commoner than previously believed. Clinical suspicion of a causative association between silica and systemic sclerosis can now be regarded as confirmed, and silica exposure has been implicated in a wide variety of CTDs. Silica seems likely to interact with several other environmental agents, notably cigarette smoking, and be affected by genetic predispositions. Where dermatological and autoantibody features of SSc occur in a man, then an additional history of silica exposure can identify a group of patients who are likely to progress and develop complications, and who should probably be treated earlier. All clinicians should be alert to this common exposure and should identify a patient’s contact with silica as early as possible. Clinicians are in a position to enable better prevention and early intervention and to offer early treatment to provide better outcomes. Dermatologists are uniquely placed to be at the forefront of these developments and work collaboratively with colleagues from other specialties in this rapidly progressing field.
Conflicts of Interest: Neither of the authors have any conflicts of interest to declare.
References
- Parks CG, Miller FW, Pollard KM, et al. Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int J Mol Sci. 2014; 15(8): 14269–14297.
- Englert HJ, Manolios N. Systemic sclerosis: new hope for an unyielding disease. Med J Aust 2009; 191 (7): 365-366.
- Jeganathan N, Sathananthan M. Connective Tissue Disease-Related Interstitial Lung Disease: Prevalence, Patterns, Predictors, Prognosis, and Treatment. Lung2020; 198, 735–759.
- Collis EL, Yule GU. The Mortality Experience of an Occupational Group Exposed to Silica Dust, compared with that of the General Population and an Occupational Group Exposed to Dust not Containing Silica. Journal of Industrial Hygiene 1933 Vol.15 pp.395-417.
- Akgun M, Araz O, Ucar EY, et al. Silicosis Appears Inevitable Among Former Denim Sandblasters: A 4-Year Follow-up Study. Chest. 2015 Sep; 148(3): 647-654.
- Martínez C, Prieto A, García L, et al. Silicosis: a disease with an active present. Arch Bronconeumol. 2010 Feb; 46(2): 97-100.
- Matar E, Yates DH, Blake L, et al. A case of complicated silicosis resulting from occupational exposure to engineered stone products – an Australian first. Med J Aust. 2017 May 15; 206(9): 385-386.
- Hoy RF, Baird T, Hammerschlag G, et al. Artificial stone associated silicosis: a rapidly emerging occupational lung disease. Occup Environ Med. 2018 Jan; 75(1): 3–5.
- Shtraichman O, Blanc PD, Ollech JE, et al. Outbreak of autoimmune disease in silicosis linked to artificial stone.Occup Med (Lond). 2015; 65(6): 444-450.
- Ronsmans S, Decoster L, Keirsbilck S, et al. Artificial stone-associated silicosis in Belgium.Occup Environ Med. 2019; 76(2): 133-134.
- Wu N, Xue C, Yu S, et al. Artificial stone-associated silicosis in China: A prospective comparison with natural stone-associated silicosis.Respirology. 2020; 25(5): 518-524.
- Rose C, Heinzerling A, Patel K, et al. Severe silicosis in Engineered Stone Fabrication Workers. Morbidity & Mortality Weekly Report, Sept 2019; MMWR 68: 813-818.
- Hoy RF, Glass DC, Dimitriadis C, et al. Identification of early-stage silicosis through health screening of stone benchtop industry workers in Victoria, Australia. Occupational and Environmental Medicine2021; 78: 296-302.
- Cohen RA, Petsonk EL, Rose C, et al. Lung Pathology in U.S. Coal Workers with Rapidly Progressive Pneumoconiosis Implicates Silica and Silicates. Am J Respir Crit Care Med. 2016 Mar 15; 193(6): 673–80.
- Schmajuk G, Trupin L, Yelin E, et al. Prevalence of Arthritis and Rheumatoid Arthritis in Coal Mining Counties of the United States. 2019; 71: 9: 1209-1215.
- Pollard KM. Silica, Silicosis and Autoimmunity. Frontiers in Immunology. 2016 March 11; 7:97.
- Pollard KM, Cauvi DM, Mayeux JM, et al. Mechanisms of Environmental Autoimmunity. Annual Review of Pharmacology and Toxicology 2021; 61(1), 135-57.
- Croissant JG, Butler KS, Zink JI, et al.Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nat Rev Mater 2020; 5, 886–909.
- Benmerzoug S, Rose S, Bounab B et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat Commun 2018; 9: 5226.
- Health & Safety Executive (UK) https://www.hse.gov.uk/lung-disease/silicosis.htm. Accessed 19.12.21 @18.50 hrs.
- Weill H, Jones R and Parkes WR. Silicosis and related diseases. Occupational Lung Disorders Third Edition 1994, Chapter 12; pp: 285-339.
- Toxicological profile for silica. US Department of Health & Human Services Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/toxprofiles/tp211.pdf accessed 27.12.21 at 7.11 pm.
- Rees D & Murray J. Silica. In Parkes’ Occupational Lung Disorders. Fourth Edition, Chapter 18; 187-205. Taylor & Francis, 2017.
- Kjoller K, Fris S, Mellenjaer L, et al. Connective tissue disease and other rheumatic conditions following cosmetic breast augmentation in Denmark. Arch Intern Med 2001; 161: 973-979.
- Yates DH, Johnson AR. Silicosis and other silica-related disorders. ERS Monograph Nov 2020; Chapter 10; 150-175. European Respiratory Society 2020.
- Leung CC, Sun-Yu IT, Chen W. Silicosis. Lancet 2012; 379: 2008-2018.
- Perret JL, Miles S, Brims F, et al. Respiratory surveillance for coal mine dust and artificial stone exposed workers in Australia and New Zealand: A position statement from the Thoracic Society of Australia and New Zealand. Respirology. 2020 Nov; 25(11): 1193-
- Wood C, Yates D. Respiratory surveillance in mineral dust-exposed workers. Breathe (Sheff). 2020 Mar; 16(1): 190632.
- Center for Disease Control (USA). https://www.cdc.gov/niosh/docs/96-112. Accessed 19.12.21 @ 19.10 hrs.
- Panchadhayaee P, Saha K, Saha I, et al. Rapidly Fatal Silicosis Among Jewellery Workers Attending a District Medical College of West Bengal, India. Indian J Chest Dis Allied Sci. 2015 Jul-Sep; 57(3): 165-71.
- Orriols R, Ferrer J, Tura JM, et al. Sicca syndrome and silicoproteinosis in a dental technician. Eur Respir J. 1997 Mar; 10(3): 731–4.
- Lescoat A, Ballerie A, Lecureur V, et al. The neglected association of crystalline silica exposure and systemic sclerosis. Rheumatology 2020; 3587-3588.
- Leso V, Fontana L, Romano R, et al. Artificial Stone Associated Silicosis: A Systematic Review. Int J Environ Res Public Health. 2019 Feb 16; 16(4): 568: 1-17.
- Blanc P, Anessi-Maseano I, Balmes JR, et al. The occupational burden of non-malignant respiratory diseases. Am J Respir Crit Care Med 2019; (11): 1312–1334.
- Preventing deaths from silicosis from sandblasting. https://www.cdc.gov/niosh/docs/92-102. Accessed 28.12.21 @20.00 hrs.
- Akgün M, Ergan B. Silicosis in Turkey: Is it an Endless Nightmare or is There Still Hope? Turk Thorac J. 2018 Apr; 19(2): 89-93.
- Ophir N, Shai AB, Korenstein R, et al. Functional, inflammatory and interstitial impairment due to artificial stone dust ultrafine particles exposure. Occ Environ Med 2019; 76: 875-879.
- Ferri C, Artoni E, Sighinolfi GL, et al. High serum levels of silica nanoparticles in systemic sclerosis patients with occupational exposure: possible pathogenic role in disease phenotypes. Seminars in Arthritis & Rheumatism 2018; 475-481.
- Lescoat A, Ballerie A, Lelong M, et al. Crystalline silica impairs efferocytosis abilities of human and mouse macrophages: implication for silica-associated systemic sclerosis. Front Immunol 2020; 219; doi: 10.3389/fimmu.2020.00219.
- Esswein EJ, Breitenstein M, Snawder J, et al. Occupational Exposures to Respirable Crystalline Silica During Hydraulic Fracturing Journal of Occupational and Environmental Hygiene, 2013; 10: 347–356.
- Vincent M. Silicose et poudre à récurer [Silicosis and scouring powder]. Rev Mal Respir. 1999 Jun; 16(3): 404-6.
- Norboo T, Angchuk PT, Yahya M, et al. Silicosis in a Himalayan village population: Role of environmental dust. Thorax. 1991; 46: 341–3.
- Berry CR, O’Brien TR, Madigan JE, et al. Thoracic Radiographic features of silicosis in 19 horses. Journal of Veterinary Internal Medicine 1991; 5248-256.
- Goodarzi M, Azizi S, Koupaei MJ, et al. Pathologic findings of anthraco-silicosis in the lungs of one humped camels (Camelus dromedarius). Journal of Camel Practice and Research 2013; 20(1): 93-96.
- Turner MT, Samuel SR, Silverstone EJ, et al. Silica exposure and connective tissue disease: an under-recognised association in three Australian artificial stone workers. Am J Respir Crit Care Med2020; 201: 378–380.
- Haustein UF, Lietzberg B. Occupational Connective Tissue Disorders. In: John S, Johansen J, Rustemeyer T, Elsner P, Maiback H (eds). Kanerva’s Occupational Dermatology. Springer, Cham. https://doi.org/10.1007/978-3-319-68617-2_29.
- Ferri C, Arcangeletti M-C, Caselli E, et al. Insights into the knowledge of complex diseases: Environmental infectious/toxic agents as potential etiopathogenetic factors of systemic sclerosis. Journal of Autoimmunity 2021; 124: 102727.
- Miller FW, Alfredsson L, Costenbader KH, et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012; 39(4): 259-271
- Industrial Injuries Advisory Council Occupational exposure to crystalline silica and its relation to connective tissue diseases: IIAC position paper 42.
- ANSES French Agency for Food, Environmental and Occupational Health & Safety. Updating knowledge on the hazards, exposures and risks associated with crystalline silica. ANSES Opinion Request No 2015-SA-0236 – Crystalline silica.
- Chifflot H, Fautrel B, Sordet C, et al. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008 Feb; 37(4): 223-35.
- Meyer A, Chifflot H, Chatelus E, et al. (2016) Brief report: spatial heterogeneity of systemic sclerosis in France: high prevalence in the northeast region. Arthritis Rheumatol 68(7): 1731–1737.
- Bergamasco A, Hartmann N, Wallace L, et al. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clinical Epidemiology 2019; 257-273.
- Bramwell B. Diffuse sclerodermia: its frequency, its occurrence in stonemasons; its treatment by fibrolysin. Edinb Med J 1914; 12: 387-401.
- Erasmus LD. Scleroderma inn goldminers in the Witwatersrand with particular reference to pulmonary manifestations, S African Laboratory and Clinical Medicine 1957; 3: 209-
- Francia A, Monarca A, Cavallot A. Osservazionis clinic-roentgenologiche soll’associzione silicosis-sclerodermia. Med Lav 1959; 50: 523.
- Sluis -Cremer GK, Hessel PA, Hnidzo E, et al. Silica, silicosis and progressive systemic sclerosis. Br J Ind Med 1985; 42: 838-843.
- Owens GR, Medsger TA. Systemic sclerosis secondary to occupational exposure. Am J Med 1988, 85, 114–116.
- Rodnan G, Benedek T, Medsger T, et al. The association of progressive systemic sclerosis (scleroderma) with coal miners' pneumoconiosis and other forms of silicosis. Ann Intern Med 1967, 66, 323–334.
- Medsger T & Benedek TG. History of skin thickness assessment and the Rodnan skin thickness scoring method in systemic sclerosis. JSRD 2019; 4(2): 83-
- Rustin MHA, Bull HA, Ziegler V, et al. Silica-induced systemic sclerosis is clinically, serologically indistinguishable from idiopathic system sclerosis. Br J Dermatol 1990; 123: 725-734.
- Gabay C, Kahn MF. Les sclerodermies masculines role de l’exposition professionelle. Schweiz Med Wochensch 1992 122: 1746-1752.
- Marie I, Gehanno JF, Bubenheim M, et al. Prospective study to evaluate the association between systemic sclerosis and occupational exposure and review of the literature. Autoimmun Rev. 2014 Feb; 13(2): 151-6.
- Rocha LF, Luppino Assad AP, Marangoni RG, et al. Systemic sclerosis and silica exposure: a rare association in a large Brazilian cohort. Rheumatol Int 2016; 697-702.
- McCormic ZD, Khuder SS, Aryal BK, et al. Occupational silica exposure as a risk factor for scleroderma: a meta-analysis. Int Arch Occup Environ Health. 2010 Oct; 83(7): 763-9.
- Marie I, Menard JF, Duval-Modeste AB, et al. (2015) Association of occupational exposure with features of systemic sclerosis. J Am Acad Dermatol 72(3): 456–
- Freire M, Alonso M, Rivera A, et al. Clinical peculiarities of patients with scleroderma exposed to silica: a systematic review of the literature. Sem Arthritis Rheumatism 2015; 45: 294-300.
- Rubio-Rivas M, Moreno R, Corbella X. Occupational and environmental scleroderma. Systematic review and meta-analysis. Clin Rheumatol. 2017 Mar; 36(3): 569-582.
- Truchetet ME, Brembilla NC, Chizzolini C. Current Concepts on the Pathogenesis of Systemic Sclerosis. Clin Rev Allergy Immunol. 2021 Sep 6.
- Patel S, Morrisroe K, Proudman S, et al. Occupational silica exposure in an Australian systemic sclerosis cohort. Rheumatology 2020; 69: 3900-3905.
- Lescoat A, Ballerie A, Lecureur V, et al. The neglected association of crystalline silica exposure and systemic sclerosis. Rheumatology (Oxford). 2020 Dec 1; 59(12): 3587-3588.
- Mostmans Y, Richert B, Badot V, et al. The importance of skin manifestations, serology and nailfold (video)capillaroscopy in morphea and systemic sclerosis: current understanding and new insights. J Eur Acad Dermatol Venereol. 2021.
- Mecoli CA, Casciola-Rosen L. An update on autoantibodies in scleroderma. Curr Opin Rheumatol. 2018 November; 30(6): 548–553.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of Scleroderma and Scleroderma-like Disorders: a comprehensive review. Clinic Rev Allerg Immunol 2017; 53: 306-336.
- Stochmal A, Czuwara J, Trojanowska M, et al. Antinuclear antibodies in systemic sclerosis: an update. Clin Rev Allergy Immunol.(2020) 58:40–51.
- Tomokuni A, Otsuki T, Sakaguchi H, et al. Detection of Anti-Topoisomerase I Autoantibody in Patients with Silicosis. Environmental Health and Preventive Medicine 2002; 7: 7–10.
- Shah AA, Rosen A, Hummers L, et al. (2010). Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis & Rheumatism, 62(9), 2787–
- Jordan S, Maurer B, Toniolo M, et al. (2015) Performance of the new ACR/EULAR classification criteria for systemic sclerosis in clinical practice. Rheumatology (Oxford) 54(8): 1454–1458.
- Avouac J, Fransen J, Walker UA, et al. EUSTAR Group. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a delphi consensus study from EULAR scleroderma trials and research group. Ann Rheum Dis2011; 70: 476–481.
- Deane K, Demouruelle M, Kelmenson LB, et al. Genetic and environmental risk factors for rheumatoid arthritis Best Pract Res Clin Rheumatol. 2017 Feb; 31(1): 3–18.
- Murphy D, Hutchinson D. Is Male Rheumatoid Arthritis an Occupational Disease? A Review. The Open Rheumatology Journal 2017; 11: 88-105.
- Willenze A, Trouw LA, Toes RE, et al. The influence of ACPA status and characteristics on the course of RA. Nat Rev Rheumatol 2012; 8(3): 144-152.
- Perry E, Kelly C, Eggleton P, et al. The lung in ACPA-positive rheumatoid arthritis: an initiating site of injury? Rheumatology 2014; 53(11): 1940–1950.
- Colinet E. Polyarthrite chronique evolutive et silcose pulmonaire. Acta Physiother Rheumatol Belg. 1953; 8: 37–
- Caplan A. Certain unusual radiological appearances in the chest of coal-miners suffering from rheumatoid arthritis. Thorax. 1953; 8: 29–37.
- Miall WE, Caplan A, Cochrane AL, et al. An epidemiological study of rheumatoid arthritis associated with characteristic chest x-ray appearances in coal-workers. Br Med J 1953; 2: 1231–6.
- Klockars M, Koskela RS, Järvinen E, et al. Silica exposure and rheumatoid arthritis: a follow up study of granite workers 1940-81. Br. Med. J. (Clin. Res. Ed.) 1987; 294(6578): 997–1000.
- Stolt P, Källberg H, Lundberg I, et al. EIRA study group Silica exposure is associated with increased risk of developing rheumatoid arthritis: Results from the Swedish EIRA study. Ann. Rheum. Dis. 2005; 64(4): 582–586.
- Stolt P, Yahya A, Bengtsson C, et al. EIRA Study Group Silica exposure among male current smokers is associated with a high risk of developing ACPA-positive rheumatoid arthritis. Ann. Rheum. Dis. 2010; 69(6): 1072–1076.
- Calvert GM, Rice FL, Boiano JM, et al. Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup. Environ. Med. 2003; 60(2): 122–129. doi: 10.1136/oem.60.2.122.
- Morotti A, Sollaku I, Franceschini F, et al. Systematic Review and Meta-analysis on the Association of Occupational Exposure to Free Crystalline Silica and Rheumatoid Arthritis Clin Rev Allergy Immunol, 2021; 5; 560(1): 81-91.
- Mehri F, Jenabi E, Bashirian S, et al. The association between occupational exposure to silica and risk of developing rheumatoid arthritis: a meta- analysis. Safety & Health at Work 2020: 11: 136-142.
- Blanc PD, Järvholm B, Torén K. (2015) Prospective risk of rheumatologic disease associated with occupational exposure in a cohort of male construction workers. Am J Med 128(10): 1094–1101.
- Wrangel O, Graff P, Bryngelsson IL, et al. Silica Dust Exposure Increases Risk for Rheumatoid Arthritis: A Swedish National Registry Case-Control Study. JOEM 2021; 63 (11): 951-955.
- Pagnoux C. Updates in ANCA-associated vasculitis. Eur J Rheumatol. 2016 Sep; 3(3): 122–133.
- Parks CG, Cooper GS. (2005) Occupational exposures and risk of systemic lupus erythematosus. Autoimmunity 38(7): 497–506 Review.
- Industrial Injuries Advisory Council. Occupational exposure to silica or asbestos and antineutrophil cytoplasmic antibodies (ANCA)- associated vasculitis. Position paper No 46. www. gov.uk/iiac; accessed 24.12.21@18.30 hrs.
- Hatman E. Rheumatological Diseases in Denim Sandblasters with Silicosis. Turk Thorac J 2020; 21(6): 446-50.
- Knight A, Sandin S, Askling J. Occupational risk factors for Wegener’s granulomatosis: a case control study. Ann Rheum Dis. 2010; 69: 737–40.
- Lane SE, Watts RA, Bentham G, et al. Are environmental factors important in primary systemic vasculitis? A case-control study. Arthritis Rheum. 2003; 48: 814–23.
- Makol A, Reilly MJ, Rosenman KD. Prevalence of connective tissue disease in silicosis (1985-2006) - a report from the state of Michigan surveillance system for silicosis. Am J Ind Med. 2011; 54: 255–62.
- Boudigaard SH, Schlünssen V, Vestergaard JM, et al. Occupational exposure to respirable crystalline silica and risk of autoimmune rheumatic diseases: a nationwide cohort study. Int J Epidemiol. 2021 Aug 30; 50(4): 1213-1226.
