Minireview on Highly Stretchable Hydrogels for Wound Healing Applications
Peter Sweeney, Kyle Vaughn, Hai-Feng Ji*
Department of Chemistry, Drexel University, Philadelphia, PA 19104, USA
Abstract
Hydrogels show great potential as biocompatible materials with a wide variety of applications. This includes, but is not limited to, wound dressings, signal detection, tissue repair, and adhesives. This minireview focuses on the recent development of highly stretchable hydrogels for wound healing applications. The hydrogels reviewed display high elasticity and self-healing properties that increase their longevity when in use.
Introduction
Hydrogels are highly absorbent, three-dimensional polymer networks. The hydrophilic chains that compose these gels are heavily cross-linked and often have adhesive capabilities. Traditional hydrogels are often used for wound healing due to their ability to create conditions for tissue restoration and their ability to adhere to human skin. Hydrogels provide a slightly moist environment that can promote better wound healing by absorbing exudate and releasing water as needed. They do not require additional glues or tape to attach to skin, reducing waste and potentially lowering costs.
Although hydrogels are of interest due to their higher durability and increased longevity, allowing them to serve as wound dressings under greater stress and for longer, wound dressings often face stressors such as stretching. These can lead to tearing and degradation over time. Many experiments in tough hydrogels have sought to solve these issues through methods such as doping traditional hydrogels with different substances. Improving the strength of the hydrogel matrix reduces the severity of wear and tear. In addition, damage is often compensated for by self-healing capabilities seen in many types of hydrogels. This review will summarize the recent improvement in this area.
Highly Stretchable and Self-adhesive Hydrogels Used in Air
Wang, et al. developed a single-reactor method of copolymerization to synthesize a dual-network hydrogel structure to improve mechanical strength1. The self-healing hydrogel structure is comprised of silk fibroin powder and polyacrylamide (SFP-PAM) (Figure 1). This combination features hydrogen bond supramolecular actions. The maximum stress and strain were measured to be 0.65 MPa and 2250%, respectively, displaying high mechanical properties. The hydrogels were capable of self-healing and showed adhesive properties. Potential applications are evident for dressing wounds and artificial skins used for signal detection on the body.
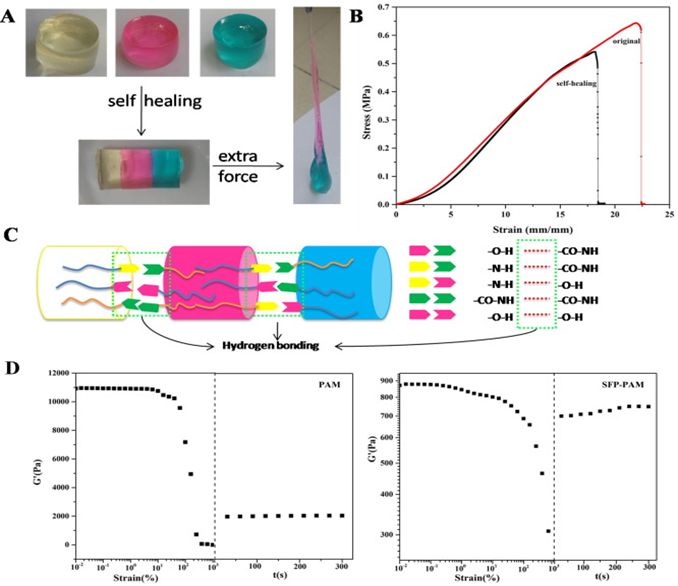
Figure 1: (A) Photographs showing the self-healing behavior; (B) stress-strain curves of the hydrogels; (C) schematic illustration of the self-healing mechanism of the hydrogel after a cut; (D) rheological test of PAM and SFP-PAM hydrogel1. (Figure with permissions from Elsevier).
Yang, et al. designed and fabricated a self-healing hydrogel adhesive capable of reversible adhesion to organic and inorganic substrates, being extended to 55 times its initial length, and self-healing within 30 seconds at ambient environment2. The hydrogel was created by processing acrylamide monomer and hyper-branched polyethylenimine polymer using ultraviolet light (Figure 2). The polyacrylamide and hyper-branched polyethylenimine (PAM-PEI) networked hydrogel is capable of repeated self-healing between 1% and 1000% strain. Additionally, the self-healed hydrogel fragments displayed good injectability which is useful for 3D-printing, indicating there are potential engineering applications in addition to the biological uses such as cartilage repair and wound closure.
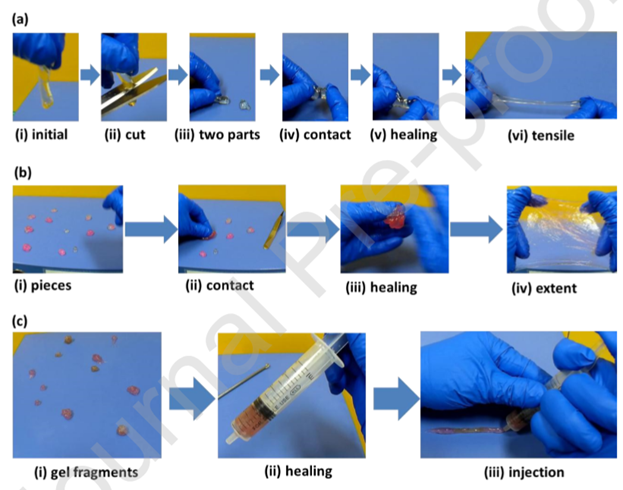
Figure 2: The demonstration of self-healing and injectable properties of the hydrogel samples (PEI: PAM = 1: 9). (a) The self-healing process of two hydrogel pieces after cutting. (i-iii) The hydrogel was cut into two parts; (iv, v) The separated pieces were brought into contact for 30 seconds; (vi) The healed hydrogel could be stretched immediately after healing. (b) The self-healing process of hydrogel fragments. (i-iii) Pieces of hydrogel healed together after contacting; (iv) The healed hydrogel could be extended in two directions. (c) The injection process of the healed hydrogel. (i-ii) The gel fragments were collected in a syringe; (iii) The healed hydrogel could be injected out of a syringe2. (Figure with permissions from Elsevier).
Hou, et al. created a mechanically elastic and self-adhesive hydrogel composed of polyurethane-poly(acrylamide) (PU-PAM) for the purpose of wound-healing3. The hydrogel is cured using ultraviolet light on a waterborne emulsion and is finished within 90 seconds. The formation of the hydrogel’s interpenetrating polymer network, consisting of physically crosslinked polyurethane trapped within a chemically crosslinked polyacrylamide network, is facilitated by the polyurethane’s ability to bridge the network. The gel displays significant ductility and stretching ability. Hydrogen bonding and electrostatic interactions enable the hydrogel to strongly adhere to skin and be removed without irritation. Animal studies indicated that the created hydrogel is biocompatible, has a notable capability for skin regeneration, and shows promise for treating burns and chronic wounds that are often challenging to treat (Figure 3). The hydrogel’s robust mechanical strength and porous structure aid in healing. The former provides protection and stabilization for wounds even under a variety of harsh conditions and the latter allows the dressing to create an ideally moist, aseptic, and breathable environment.
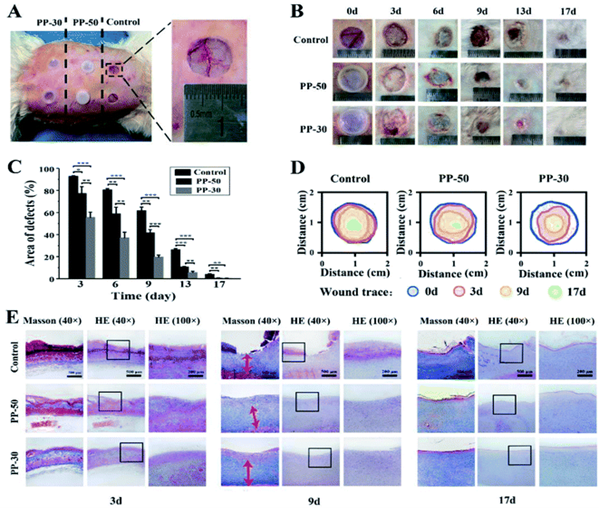
Figure 3: In vivo wound healing experiments. (A) Skin defects of a rabbit and application of PU-PAM hydrogels; (B) visual inspection of the healings skin surface after surgery; (C) quantitative analysis of skin healing after surgery (***p < 0.001, **p < 0.01, *p < 0.05); (D) traces of wound-bed closure for each treatment; (E) defected skin tissue with HE and Masson staining after 3, 9, 17 days, respectively3. (Figure with permissions from RSC).
Xue, et al. developed a hydrogel composed of quaternized chitosan-Matrigel-polyacrylamide (QCS-M-PAM) (Figure 4) with multiple functions that was investigated extensively in vitro and in vivo4. The morphology, swelling ratio, mechanical properties, antimicrobial properties, hemostatic performance, and biocompatibility were examined. These investigations showed that the hydrogel displayed excellent stretchable and compressive properties that bore similarity to human skin, good adhesive properties, and low toxicity to cells. The hydrogel displayed the highest fracture strain of those investigated with a value of 1210%. The tensile strength was recorded to be 118.3 kPa. In vivo molecular testing indicated that the produced hydrogel has potential to significantly enhance the healing of wounds, the deposition of collagen, and regenerate skin by encouraging anti-inflammatory factors and discouraging proinflammatory factors. These properties show promise for wound-dressings for full-depth skin defects.
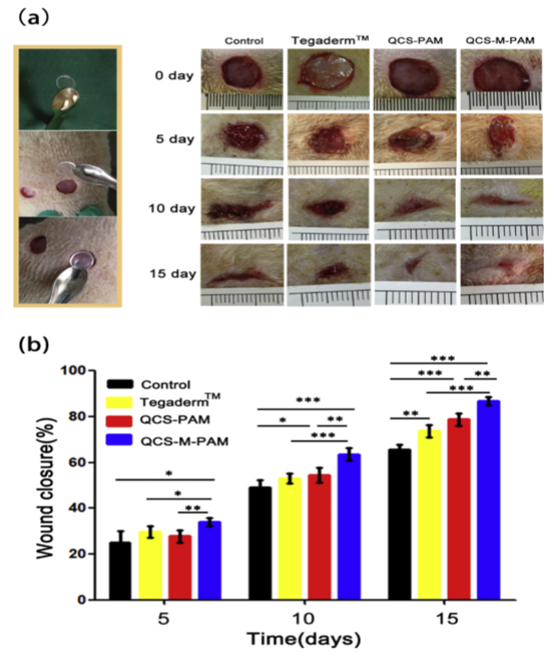
Figure 4:(a)Application of the hydrogels in the full-thickness skin excision and representative photographs of the wounds on the 0th, 5th, 10th and 15th day dressed by the Vaseline gauze (control), commercial film dressing (Tegaderm™), QCS-PAM hydrogel and QCS-M-PAM hydrogel (b) wound closure at different timepoints for the Vaseline gauze (control), commercial film dressing (Tegaderm™), QCS-PAM hydrogel and QCS-M-PAM hydrogel groups. Data are given as mean ± SD (n=8). *p < 0.05, **p < 0.01, ***p < 0.001.4 (Figure with permissions from Elsevier).
He, et al. demonstrated a strategy to improve the adhesive capabilities of hydrogels by incorporating a microgel with a positive charge (MR) into the hydrogel’s network of poly(acrylic acid) (PAAc), polyacrylamide (PAM), and polydopamine (PDA) to create the MR/PAAc–PAM–PDA hydrogel5. This was motivated by the natural adhesive mechanism used by mussels. This method of adhesion showed good bondage to organic surfaces such as polythene, polytetrafluoroethylene, and rubber, and inorganic surfaces such as ceramics, glass, and copper in addition to biological tissues such as lung, kidney, heart, liver, and hogskin (Figure 5). The adhesion to glass substrates were 8 times higher for this hydrogel than hydrogels using only catechol moieties, meaning the doping of the hydrogel significantly improved its adhesive abilities. The hydrogel is capable of rapid self-healing and stretching significantly. The latter is due to the noncovalent interactions between the matrices of the hydrogel and the microgel. The hydrogel’s tensile strength was recorded to be about 160 kPa at the fracture strain of about 80%.
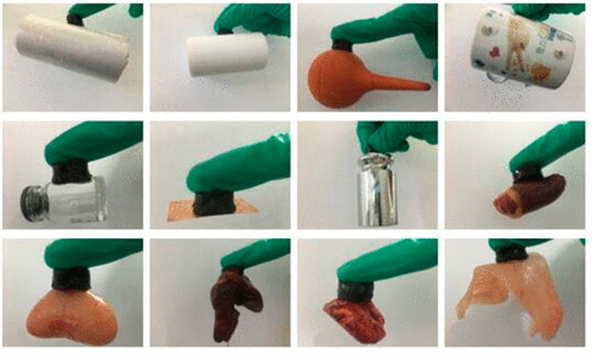
Figure 5: Adhesion of hydrogel (column hydrogels with a diameter of 16 mm, a thickness of 10 mm, and a weight of about 3 g) to various substrates: polythene, PTFE, rubber, ceramics, glass, copper, weight of 500 g, heart, liver, lung, kidney, and hogskin (from left to right and then from top to bottom) 5. (Figure with permissions from ACS).
Qu, et al. designed a self-healing injectable composite of micelle and hydrogel with multiple functions as wound dressings for joint skin damage6. This composite was created by creating a mixture of quaternized chitosan and Pluronic®F127 terminated with benzaldehyde (QCS/PF) (Figure 6). The composite has inherent antibacterial properties and biodegrades under certain pH conditions, making them useful for wound dressing. The composite shows similar compressive and elastic properties to human skin due to a Young’s modulus of 21.5 kPa to 37.5 kPa, good adhesiveness, and self-healing capabilities. It is biocompatible and efficiently stifles bleeding. Curcumin, after being included in the hydrogel, displayed antioxidant and pH-responsive capabilities. These curcumin-loaded hydrogels significantly increased the rate of wound healing in vivo, showing greater granulation tissue thickness, collagen deposition, and promoted vascular endothelial development in a full-depth skin defect model.
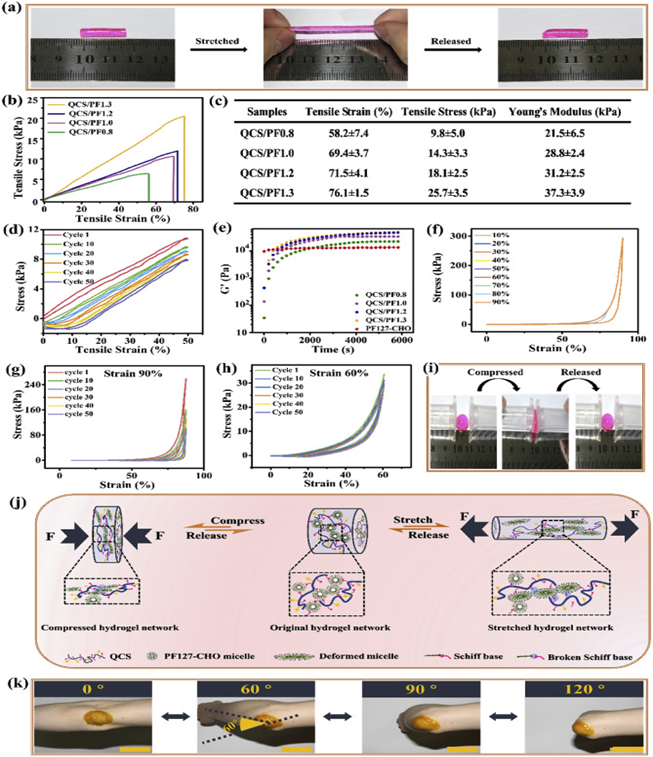
Figure 6: Mechanical characterizations of QCS/PF hydrogels. (a) Photographs of stretching and release process of QCS/PF1.0 hydrogel; (b) Stress−strain profile of hydrogels; (c) Summary of mechanical properties; (d) Cyclic tensile tests of QCS/PF1.0 hydrogel at a strain of 50% under the deformation rate of 30 mm/min; (e) Rheological behavior of hydrogels; (f) Compression and tension stress strain curves of QCS/PF1.0 hydrogel at strains from 10% to 90%; Compression and tension stress strain curves of QCS/PF1.0 hydrogel at strains of (g) 90% and (h) 60%; (i) Photographs of compression and release process of QCS/PF1.0 hydrogel; (j) The schematic diagram of the proposed mechanism for stretchable and compressive QCS/PF hydrogels; (k) Photograph of Cur-QCS/PF1.0 hydrogels that were applied on the human elbow. Scale bar: 5 cm6. (Figure with permissions from Elsevier).
Jing, et al. synthesized a new hydrogel by assimilating polydopamine-coated talc nanoflakes into a hydrogel composed of polyacrylamide (DTPAM) inspired by the method by which mussels adhere to surfaces (Figure 7)7. Talc was embedded with dopamine molecules which were then oxidized. This improved the dispersal of talc and preserved the catechol groups within the hydrogel’s structure. The resultant hydrogel displayed exceptional elasticity, extending by over 1000% with a greater than 99% recovery rate. It has strong adhesive properties when attached to a variety of substrates, including human skin, and outperformed commercial adhesives such as glue sticks and double-sided tape even after dehydration. It displayed impressive self-healing capabilities without loss of its mechanical properties and was extremely biocompatible. The hydrogel shows promise as a high-sensitivity strain sensor. At 1000% strain, a gauge factor of 0.693 was recorded. It was adept at monitoring various human motions such as taking a deep breath and bending a finger, knee, or elbow. This indicates that it has favorable properties for utilization in human-compatible biological devices.
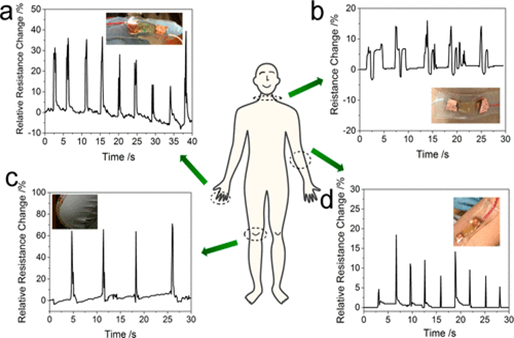
Figure 7: Demonstration of real-time human motion detection using the DTPAM hydrogel strain sensor directly attached to a human body. (a) Signal detected for the rapid bending and straightening of a human index finger. (b) Signal detected for a human’s deep breath. (c) Signal detected for the rapid bending and straightening of a human knee. (d) Signal detected for the rapid bending and straightening of a human elbow 7 (Figure with permissions from ACS).
Parameters, such as healing time, adhesion force, etc that affect the mechanical properties of the hydrogels are listed in Table 1.
Table 1: The tensile strength, fracture strain, healing time, and adhesive strength of the tough hydrogels
|
Hydrogel |
Tensile strength |
Fracture strain |
Self-Healing time |
Adhesive strength |
Antimicrobial properties |
|
SFP-PAM |
650â¯kPa |
2250% |
<6 h |
-- |
-- |
|
PAM-PEI |
-- |
>5000% |
30 s |
7 to 90 kPa |
-- |
|
PU-PAM |
400 kPa |
2560% |
-- |
3 to 15 kPa |
-- |
|
QCS-M-PAM |
118.3 kPa |
1210% |
-- |
1.3 to 2.7 kPa |
Antibacterial, antifungal |
|
MR/PAAc–PAM–PDA |
160 kPa |
80% |
“A few seconds” |
4 kPa |
-- |
|
QCS/PF |
-- |
<80% |
-- |
4.4 to 6.1â¯kPa |
Antibacterial |
|
DTPAM |
-- |
>1000% |
-- |
15.2 kPa |
-- |
The tensile strength of SFP-PAM being the highest at 650 kPa is likely due to the hydrogen bonding between the carbonyl and C-N groups of SFP and PAM. Each subunit contains many of each group, likely allowing a strong network of hydrogen bonds to be established. This would also contribute to its relatively high fracture strain of the hydrogels reviewed. The adhesive strength of PAM-PEI, 90 kPa to iron, is the highest likely due to the ability of the carbonyl and amine groups of the hydrogel to form metal complexes with the substrate along with the hydrogen bonding these groups facilitate. The self-healing time of MR/PAAc–PAM–PDA was the lowest most likely due to the large amount of reversible chemical cross-linking of the hydrogel matrix.
Adhesion Under Water or in Wet Conditions
Luo, et al. constructed a silk-fibroin (SF) and tannic acid (TA)-derived multifunctional hydrogel adhesive (Figure 8). These showed high extensibility, up to 32000%, and self-healing abilities. In water, they could create a watertight seal and adhere to surface underwater. Under moist conditions, the maximum adhesive strength to porcine skin tissue was 69.4 ± 5.3 kPa. They also showed biocompatibility and antibiotic properties. The high extensibility is likely due to the hydrogen bonds between the silk-fibroin and tannic acid chains in water. These hydrogels showed potential for medical applications including tissue adhesives and bioelectronics8.
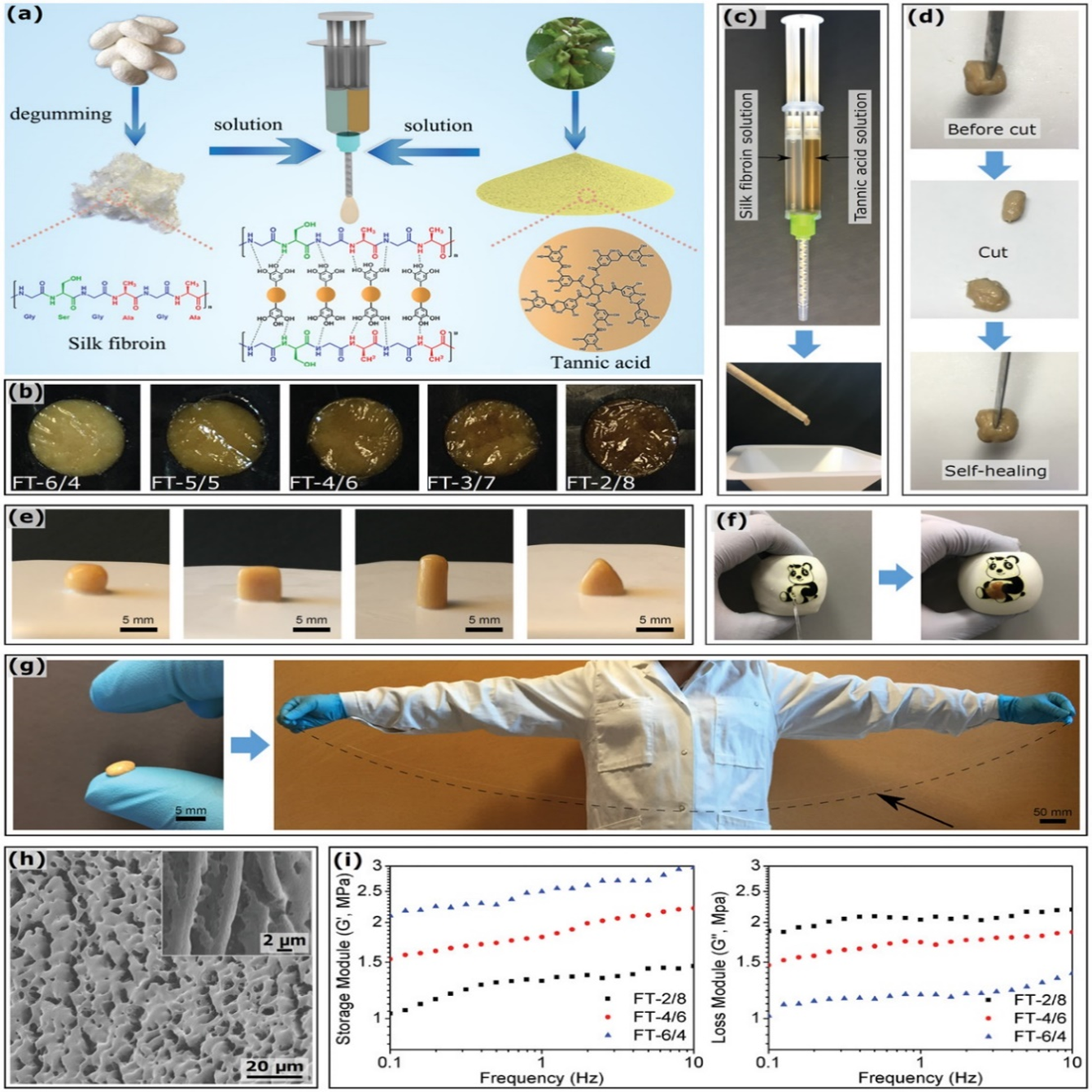
Figure 8: Schematic of the FT hydrogel adhesive formation. b) Color of FT hydrogel adhesives according to the SF/TA ratio. c) Preparation of FT hydrogel adhesives by mixing an SF solution and a TA solution in a twin-barreled syringe, and efficiently ejecting from the static mixer. d) Self-healing property of the FT hydrogel adhesive. The initial sample was cut into two pieces and then brought together again; the self-healing process instantaneously yielded a recovered adhesive (Group FT-4/6 is given as an example). e) FT hydrogel adhesive molds; they can be molded into various shapes (Group FT-5/5 is given as an example) and support their own weight. f) Waterproof property of FT hydrogel adhesives: they can patch the balloon and instantly seal out water to create a flexible and watertight barrier (Group FT-2/8 is given as an example). g) High extensibility of FT hydrogel adhesives: a Group FT-6/4 sample was stretched up to 32000%. h) Microstructure of FT hydrogel adhesives: cryo-SEM images of a Group FT-6/4 sample before and after (insert) manual stretching. i) Rheological behavior of FT hydrogel adhesives; the modulus of Groups FT-2/8, FT-4/6, and FT-6/4 are shown for different frequencies8.(Figure with permissions from RSC).
Zhu, et al. synthesized a poly(glutamic acid) (PGA), lysine (Ly) And small organic molecule (OSM) hydrogel with extraordinary swelling properties, capable of expanding 2750% in PBS and almost 10000% underwater. Using 10% diethylene glycol increased the elongation from 238% to 2705% (Figure 9). Cell and animal experiments were performed that indicated that the hydrogels had good biocompatibility and bioactivity, showing potential for clinical applications. Interstitial phases of small organic species were shown to contribute to the evolution of stretchable and self-healing properties in hydrogel networks9.
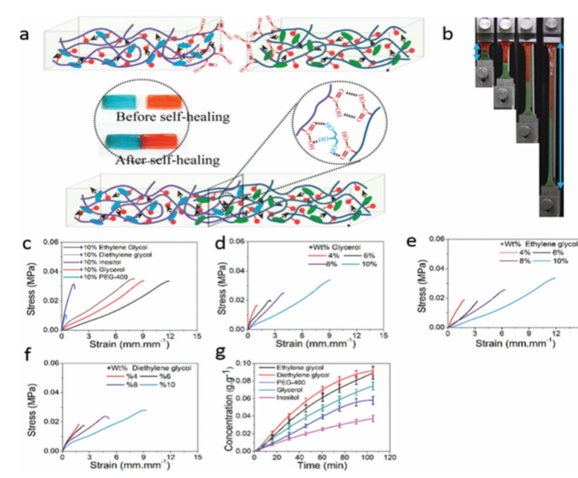
Figure 9: (a) Illustration of the self-healing process of hydrogels. (b) Images of the self-healed sample during the tensile test. (c) Tensile stress–strain curves of the self-healed hydrogels with interstitial phases with the weight percentage of 10%. (d–f) Tensile stress–strain curves of the self-healing hydrogels with ‘‘interstitial phase’’ glycerol (c), ethylene glycol (d) and diethylene glycol (e) with the weight percentages of 4%, 6%, 8% and 10%. (g) Diffusion rate for small organic molecules through dialysis sacks with a wide flat of 34 mm and a MWCO of 1000 Da at different time points9. (Figure with permissions from RSC).
Zhu, et al. developed an injectable composite hydrogel by using a unique method for crosslinking silica nano-particles (SiNP) with glycol chitosan (GC) (Figure 10). Gelation was performed in an aqueous solution where the GC segments were incorporated with SiNP. Rheology tests indicated that the moduli of the hydrogel was affected by the composition of the constituents, the volume of the nano-particles, and the configuration of the polymers. The adhesiveness of the hydrogel was tested using mouse skin and it displayed a lap-shear stretching force of 90 kPa. The hydrogel also showed good injectability, allowing for administration directly to the wound site where it could easily adhere and fill in the damaged area. It was also capable of carrying proteins and cells, which through the mouse model showed the capability to treat full-thickness skin defects and grow hair follicles and microvessels, reducing potential scar formation. The adhesive capabilities of the hydrogel weakened significantly when underwater. However, resistance to water is not a necessity for dressing wounds10.
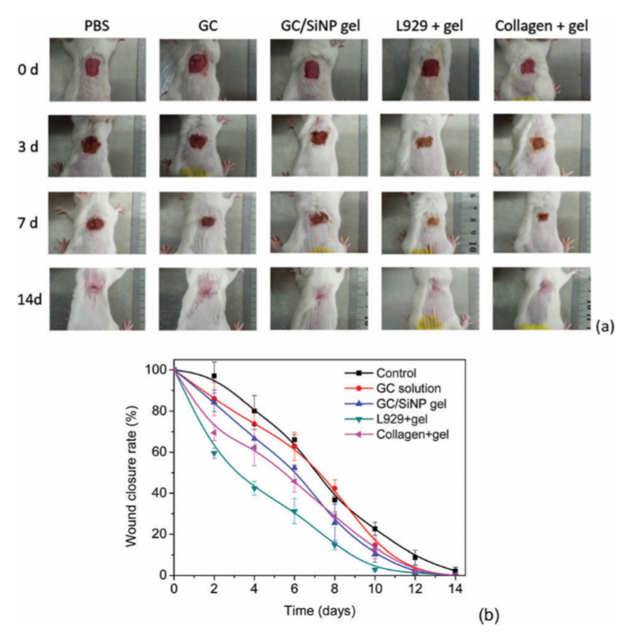
Figure 10: (a) Images of wounded animals after treatment with PBS, 100mg mL-1 GC solution, a GC100/SiNP10 hydrogel, an L929 loaded GC100/SiNP10 hydrogel and a collagenloadedGC100/SiNP10 hydrogel. (b) Wound closure rate for animals treated with different formulations. The closure rate of the L929 loaded hydrogel and the control groups is significantly different on each day from day 2 to day 12 (p < 0.05)10. (Figure with permissions from RSC).
Table 2: The tensile strength, fracture strain, healing time, and adhesive strength of the tough hydrogels
|
Hydrogel |
Tensile strength |
Fracture strain |
Self-Healing time |
Adhesive strength |
Antimicrobial properties |
|
SF-TA |
-- |
-- |
“Instantaneously” |
69.4 ± 5.3 kPa |
Antibiotic |
|
PGA-Ly-OSM |
72 to 112 kPa |
691 to 2705% |
-- |
-- |
-- |
|
SiNP-GC |
-- |
-- |
-- |
90 kPa |
Antibacterial |
Conductive Gel for Skin, and by Electrical Stimulation
Han et. al. devised a novel method of synthesizing a transparent, adhesive and conductive hydrogel that can be used in bioelectronics (Figure 11)11. The polyacrylamide (PAM) gel was synthesized with an addition of Pyrrole (Py) and dopamine (DA) nanoparticles which, after three days, polymerized to nanofibrils. Typically, PPy gels are good conductors, but are hydrophobic and opaque. Han et. al. found that doping the gel with PDA increased hydrophilicity, transparency, and conductivity, while maintaining elasticity. The conductivity of a PPy-PAM gel was less than 6 S/m, while the PDA doped PPy-PAM gel’s conductivity measured around 12 S/m. While the gel allows for visible light to pass through, it scatters ultraviolet light, and was shown to protect skin cells from UV exposure better than a purely PAM gel. When compared to a strictly PAM gel, the adhesion strength of a 0.6 wt. % PDA-PPy-PAM gel is nearly doubled (from about 13 kPa to a about 24 kPa). The PPy-PDA gel also showed greater cell culture affinity than PAM gel over a period of seven days, showing promise for use as a wound dressing. Considering the most obvious application of these types of hydrogels is in wearable electronic devices, such a large increase in conductivity while maintaining the gel’s other favorable qualities establishes a PDA-PPy-PAM gel’s utility.
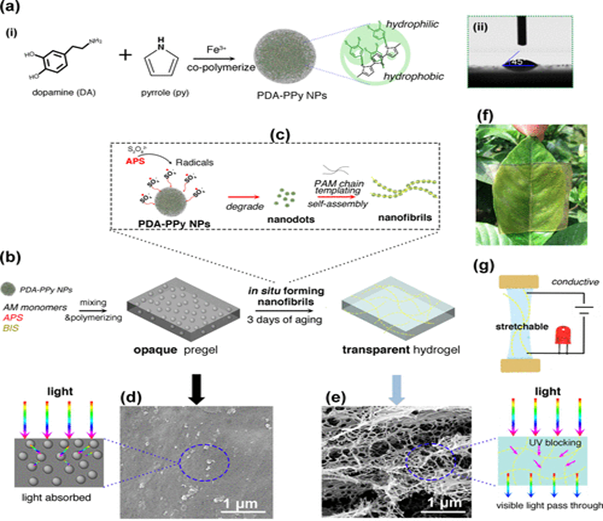
Figure 11: Transparent, conductive, stretchable, and adhesive hydrogel by in situ formation of PDA–PPy nanofibrils. (a-i) Formation of hydrophilic PDA–PPy NPs. (a-ii) PDA–PPy NPs with highly hydrophilic properties. (b) Opaque pregel was formed after AM monomers polymerized in the suspension of the PDA−PPy NPs, and a transparent hydrogel was obtained after 3 days of aging. (c) In situ formation of PDA–PPy nanofibrils. During the aging process, APS generated radicals that continuously broke down the PDA–PPy NPs into nanodots, which self-assembled to form nanofibrils under the templating effect of the PAM chains. (d) SEM image of PDA–PPy NP-embedded opaque pregel, which absorbed light. (e) SEM image of nanofibrils in the transparent hydrogel, which allowed visible light to pass through while blocking UV irradiation. (f) Representative photo of the transparent hydrogel covering on a leaf. (g) Hydrogel was stretchable and conductive. (Figure with permissions from ACS).
Kai et. al. developed a bioelectric plaster out of a gellan gum/PAM double-network hydrogel, redox enzyme electrodes, and an elastic resistor12. They tested wound healing effects of an applied current on an acute wound of a mouse (Figure 12). Three groups of mice, A, B, and C were given circular wounds and allowed to heal openly, with a hydrogel, and with a bioelectric plaster for each respective group for 7 days. Group B healed the slowest while groups A and C healed at nearly the same rate. The sections of the healed skin were stained with eosin and hematoxylin and examined under a microscope. The authors observed moist healing, a smoother healing with less scarring, in groups B and C, but observed contracture of the epidermis in group A which is indicative of a less smooth healing process. Kai et. al. concluded that the bioelectric plaster they developed combined the healing speed benefits of electric stimulation with the benefits of moist healing, making it a promising wound dressing for both acute and chronic wounds.
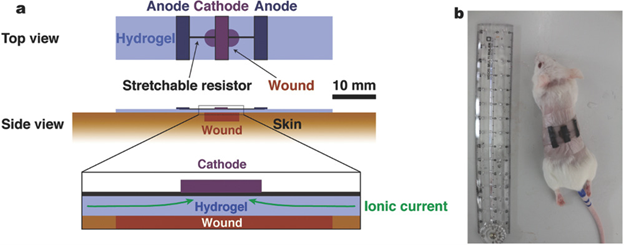
Figure 12: a) Schematic structure of a bioelectric plaster. b) A photograph of the bioelectric plaster applied on a wound of a live mouse skin. (Figure with permissions from Wiley).
Lin et. al sought to create a programmable wound dressing capable of delivering different drugs or amounts to different areas of a wound based on sensors placed in the wound dressing13. The design for the device included a hydrogel, embedded wires, rigid and flexible chips, and drug delivery channels (Figure 13). Between polyethylene glycol (PEG) alginate and PAM alginate hydrogels, the latter was chosen for its ~21x greater stretchability and greater fracture toughness (~9000 J/m2). The authors integrated titanium wires functionalized with trimethoxysilyl propylmethacrylate (TMSPMA) by casting the pre-gel around sinusoidally deformed wires. Due to the TMPSMA coating, the wires were fully integrated into the hydrogel network and could be deformed more than 10,000 times and maintain roughly their original resistance. A thin glass slide treated with oxygen plasma is covalently bound to a rigid polydimethylsiloxane (PDMS) chip in order to adhere the PDMS chip to the hydrogel network. Without the slide, the chip can be plucked from the hydrogel, the same being true if the slide is not treated and bound to the chip. Lin et. al combined both these breakthroughs and added diffusive reservoirs and non-diffusive channels to deliver drugs to the reservoir to create a smart wound dressing, capable of sensing temperature and sustaining drug release at critical areas.
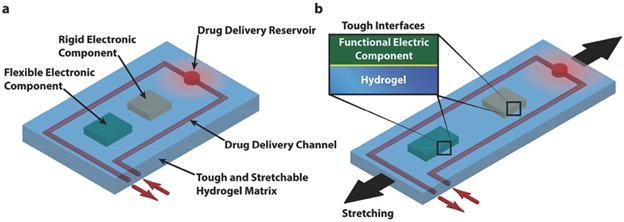
Figure 13: Schematic illustration of the design of stretchable hydrogel electronics and devices. a) Functional electronic components such as conductors, microchips, transducers, resistors, and capacitors are embedded inside or attached on the surface of the hydrogel. Drug-delivery channels and reservoirs are patterned in the hydrogel matrix, and they can diffuse drugs out of the hydrogel to give programmable and sustained release of drugs. b) As the hydrogel electronic device is stretched, flexible electronic components can deform together with the device but rigid components will maintain their undeformed shapes, which requires robust interfaces between electronic components and hydrogel matrix. (Figure with permissions from Wiley).
In his case study, Powell oversees the care of ‘Mrs. B’, an 88-year old woman who suffers from a chronic leg ulcer lasting for 10 months14. Mrs. B suffered from excessive pain from the wound, which was copiously exuding a thick slough that was swabbed and shown to contain pseudomonas and proteus bacteria. Because of the thick exudate, conventional gauze bandages were avoided as they would saturate and harden with fluid. A hydrogel dressing branded as Actiformcool® was chosen as the primary dressing because Mrs. B attributed past bandages to increased pain. 10 days into the Actiformcool treatment the wound was nearly fully debrided, and after 6 weeks showed signs of wound reduction. The dressings were changed twice weekly and healing progress was tracked for 12 weeks total. After the first 6 weeks, the wound healing stagnated, so the dressing was changed to Suprasorb X / PHMB. Suprasorb X is a hydro balance dressing that can both absorb exudate and give moisture to aid in healing. PHMB (polyhexamethylene biguanide) is an antimicrobial that is non-cytotoxic to human cells. After 2 applications the wound fully healed, and Mrs. B was seen for follow-up 4 and 12 weeks after treatment.
Conclusion
Tough hydrogels show great potential for numerous healthcare applications. Table 3 summarized tough hydrogels that have been used for wound healing. These hydrogels would likely be useful as wound dressings and in theory could be integrated into current commonly used consumer bandages. The capability of strong adhesion to human tissue indicates that the hydrogels may adhere better than typical adhesive bandages. In addition, the toughness and self-healing properties of these hydrogels would likely create more durable and longer-lasting wound dressings. The antimicrobial properties of some hydrogels would also provide a benefit over traditional bandages as the hostile environment for microbes would lower the chance of infection. When designed with channels, these hydrogels could be used to deliver drugs to the site of wounds, some may even be injected to better form to the shape of a wound to seal it off from exposure to the outside. Because of the biocompatibility of these hydrogels, they can be used in wounds, though it is likely that more research must be done to assure they are as safe as possible. Overall, tough hydrogels have many potential uses in clinical applications due to their improvements over conventional bandages.
On the other hand, as shown in Table 3, the drawbacks of these hydrogels have not been fully investigated. It is not fully studied whether they might have similar flaws as those commercial hydrogels, such as creating a fibrin clot or the risk of transfer of blood diseases. Furthermore, the monomer residues have not been evaluated, such as the percentage of untreated monomers, whether they could leach out of the hydrogel etc. Although these highly stretchable hydrogels have showed promise for found healing, further studies are necessary to understand the adequacy of these hydrogels for wound healing.
Table 3: Summary of the tough hydrogels
|
Hydrogel |
Applicability |
Advantages |
Drawbacks |
|
SFP-PAM |
Wound dressing, artificial skins, signal detection on the body |
High fracture strain, high, high tensile strength, |
Slow healing |
|
PAM-PEI |
Reversible wet adhesion, 3D-printing, cartilage repair, wound closure |
High fracture strain, high tensile strength, fast healing, high adhesive strength, adheres to organic and inorganic substrates, good injectibility |
-- |
|
PU-PAM |
Treating burns and chronic wounds, protection and stabilization for wounds under harsh conditions |
Elastic, self-adhesive, quick curing, ductile, stretchable, strong adherence to skin, removal without irritation, biocompatible, porous structure, aseptic, breathable |
-- |
|
QCS-M-PAM |
Wound dressing, enhancing wound healing, depositing collagen, encouraging anti-inflammatory factors and discouraging proinflammatory factors |
Antibacterial, antifungal, highly stretchable and compressible, low cytotoxicity |
Somewhat low adhesive strength |
|
MR/PAAc–PAM–PDA |
Would dressing |
Rapid healing, bonds well to organic and inorganic surfaces, stretchable |
Low fracture strain, low adhesive strength to some substrates |
|
QCS/PF |
Wound dressing for joint skin damage, stifling bleeding, promoting collagen deposition and vascular endothelial development |
Injectable, self-healing, antibacterial, biodegradable, pH-responsive |
Low fracture strain, |
|
DTPAM |
Human-compatible biological devices, human body activity sensors |
Antibacterial, high elasticity, adheres to many substrates, highly biocompatible |
-- |
|
SF-TA |
Watertight sealing, wound dressing, waterproof patching |
Rapid healing, antibiotic, underwater adherence, biocompatible, high extensibility |
-- |
|
PGA-Ly-OSM |
Wound dressing |
High fracture strain, good biocompatibility and bioactivity, self-healing, extraordinary swelling capabilities |
Low tensile strength |
|
SiNP-GC |
Wound dressing, direct administration to wound sites, filling in wounds, treatment of full-thickness skin defects, grow hair follicles and microvessels |
Antibacterial, good injectability, reduces scar formation |
Adhesion weakened significantly underwater |
|
PDA-PPy-PAM |
UV-protection, transparent electronic skins, wearable electronic devices, wound dressing |
Higher conductivity, transparent to visible light |
-- |
|
Gellan gum/PAM |
Chronic and acute wounds, circular wounds, wound dressing |
Bioelectric, smoother healing, less scarring, improved healing speed |
Uses an applied current |
|
PEG-PAM |
Drug delivery, sensing temperature, sustaining drug release at critical areas |
Programmable drug delivery channels, maintains resistance after many deformations, highly stretchable |
-- |
|
Suprasorb X / PHMB |
Leg ulcers, moisturizing wounds |
Antimicrobial, non-cytotoxic |
|
References
- Wang C, Du Y, Chen B, et al. A novel highly stretchable, adhesive and self-healing silk fibroin powder-based hydrogel containing dual-network structure. Mater. Lett. 2019, 252, 126-129.
- Yan Y, Xu S, Liu H, et al. A multi-functional reversible hydrogel adhesive. Colloids Surf. A: Physicochem. Eng. Aspects, 2020, 593, 124622.
- Hou Y, Jiang N, Sun D, et al. A fast UV-curable PU-PAAm hydrogel with mechanical flexibility and self-adhesion for wound healing. RSC Adv., 2020, 10, 4907-4915.
- Xue H, Hu L, Xiong Y, et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302.
- He X, Liu L, Han H, et al. Bioinspired and Microgel-Tackified Adhesive Hydrogel with Rapid Self-Healing and High Stretchability. Macromol. 2019, 52, 72-80.
- Qu J, Zhao X, Liang Y, et al. 2018. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials, 2018, 183, 185-199.
- Jing X, Mi H, Lin Y, et al. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interf. 2018; 10: 20897-20909.
- Luo J, Yang J, Zheng X, et al. A Highly Stretchable, Real-Time Self- Healable Hydrogel Adhesive Matrix for Tissue Patches and Flexible Electronics. Adv. Healthc. Mater. 2020, 9: e1901423.
- Zhu S, Wang J, Yan H, et al. An injectable supramolecular self-healing bio-hydrogel with high stretchability, extensibility and ductility, and a high swelling ratio. J Mater Chem B, 2017, 5, 7021-7034.
- Zhu F, Wang C, Yang S, et al. Injectable tissue adhesive composite hydrogel with fibroblasts for treating skin defects. J Mater Chem B, 2017, 5, 2416-2424.
- Han L, Yan L, Wang M, et al. Transparent, Adhesive, and Conductive Hydrogel for Soft Bioelectronics Based on Light-Transmitting Polydopamine-Doped Polypyrrole Nanofibrils. Chem. Mater. 2018, 30, 5561-5572
- Kai H, Yamauchi T, Ogawa Y, et al. Accelerated Wound Healing on Skin by Electrical Stimulation with a Bioelectric Plaster. Adv. Healthc. Mater. 2017, 6. doi: 10.1002/adhm.201700465.
- Lin S, Yuk H, Zhang T, et al.Stretchable HydrogelElectronics and Devices. Adv. Mater. 2016, 28, 4497-4505.
- Gail P. Hydrogel sheet dressings and short-strech cohesive bandaging: case study. Brit. J. commun. nurs. 2010, 15, S42, S44-6.
