Superficial Radiotherapy: Long Term Follow-Up of Highly Selected Basal and Squamous Cell Carcinomas in Skin Cancer Patients
Simon J. Madorsky, MD*, Orr A. Meltzer, BS, Alexander Miller, MD
Skin Cancer and Reconstructive Surgery Center (SCARS Center) at 180 Newport Center Drive, Suite 158, Newport Beach, CA 92660
Abstract
Superficial radiotherapy (SRT) treatment for non-melanoma skin cancer has been reported to yield variable cure rates. When patients are highly selected, adequate margins of treatment are chosen, and hypofractionation is avoided, cure rates of SRT can approach that of Mohs surgery.
The objective of this study is to evaluate long term results of our center’s SRT selection criteria and define proper decision-making parameters of optimal candidates for treatment, and to review the literature. A retrospective chart analysis was done of all SRT cases from 2012-2018. Location, size, type and depth of the treated tumors were defined. Treatment energy, fractionation, and radiation field size were documented. Recurrences and complications were analyzed. Of 131 treated lesions treated, head and neck lesions (105, 80%) were the most common location, primarily on the lower nose (60, 46%). Of 122 lesions analyzed for recurrence, 2 (1.6%) recurred, with a mean follow-up time of 5 years. Acute ulcerations in 29 (28%) head and neck lesions, 5 (63%) trunk lesions, and 9 (50%) leg lesions occurred. Delayed ulcerations occurred in 5 (28%) leg lesions. In conclusion, when patients are highly selected, long-term SRT cure rates up to 98% can be achieved.
Introduction
After the discovery of X-rays in 1895 by Wilhelm Roentgen1, the first successful treatment of malignant skin lesions (rodent ulcer, i.e. basal cell carcinoma) was reported by Thor Stenbeck and Tage Sjogen of Sweden in 1899.2 By 1932, Henri Coutard, working with the Radium Institute in Paris and building upon discoveries of his colleague Claude Regaud, developed the first protracted fractionated course of radiation for head and neck cancers – the concept still employed today.3,4 In the 1960's and early 1970’s, skin cancer radiotherapy was practiced widely by dermatologists. In some areas, half of dermatology practices used X-ray machines.5,6 By 1980’s, the practice began to decline and virtually disappeared over the next 20 years.7,8 As Mohs surgery gained in popularity, it supplanted office-based radiation therapy. Over the last 10 years office based superficial radiotherapy (SRT) has had a limited resurgence, with newer X-ray machines having energies between 50-100 kVp. The acceptance of the newer radiation technologies in the United States has been tempered by limited reimbursement and high cost of equipment and maintenance. Compared to the published 98-99% cure rate for Mohs surgery, reports for superficial radiation have shown recurrence rates from 4% to 16%.9-12 However, our literature review and 10-year experience suggests that highly selected patients and treatment regimens can achieve cure rates that approach those of Mohs surgery. In this study, we retrospectively analyzed 131 lesions treated with SRT treatment for non-melanoma skin cancers performed by the senior author.
Materials and Methods
Study design
IRB exemption was received from St. Joseph Health Center for Clinical Research. A retrospective medical records analysis was done on all patients who received superficial radiotherapy for basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) at our center from 2012 to 2018. Information was gathered on the treatment energy, fractionation, tumor location, size, and type, and radiation field size. Indications, recurrences, and acute and delayed complications were also noted. Follow up information was gathered at regular intervals with chart reviews and direct patient communication. Patients who received SRT for treatment of keloids or as adjuvant postoperative therapy were excluded from analysis. Informed consent was obtained from patients with published photos. Recurrence was determined to be present if the cancer recurred within 2 mm of the radiation field edge. Of the 131 BCCs and SCCs treated, 122 had documented follow-up of one year or greater. Nine had less than a year of follow-up and were excluded from recurrence analysis, mostly due to patients expiring.
Patient selection
Only superficial non-melanoma skin cancers, particularly BCCs and SCCs, were selected as candidates for SRT. Clinical presentation of less than 5 mm thickness estimated by a physical examination was deemed appropriate for SRT treatment. Additionally, since a flat dose profile beam was used, the lesions eligible for SRT treatment were either relatively flat or could be flattened with shields and applicators. Lesions greater than 6 cm were not deemed candidates for SRT due to the penumbra effect of the treatment field with 15 cm SSD (source to skin distance). Those patients were referred out to radiation oncology centers with higher energy equipment and larger SSD. SRT was recommended for patients in lieu of a more complicated reconstructive surgical plan (nose, large lesion) or where surgical removal risked injury to the temporal branch of the facial nerve. Patients with multiple simultaneous carcinomas were candidates for synchronous SRT treatment of several lesions. Exclusion criteria included carcinomas of the eyelid or lips and full thickness carcinoma involvement over the bone. Age was not a selection criterion.
Treatment administration
Our center has used both SRT machines commercially available in the USA: SRT-100 (Sensus Healthcare, Boca Raton, FL) used in 2012 for the first 24 lesions, and Xstrahl 100 (Gulmay Inc, Suwanee, GA) used for the remainder of lesions after 2012. The energy selection between 50 and 95 kV was based on the estimated maximum depth of the tumor: 107 lesions were treated with 50 kV, 2 lesions with 70 kV, 19 lesions with 80 kV, and 3 lesions with 95 kV. The energy chosen had to treat the depth of the tumor to at 80% of delivered dose (D80)13. For example, 80 kV was chosen to treat a 6 mm tumor depth with D80 of 6mm of Xstrahl 100’s 3 cm field. SSD for both machines was 15 cm. Applicator sizes used included 1 case of 1.5cm, 12 cases of 2cm, 7 cases of 2.5cm, 22 cases of 3cm open, 53 cases of 3cm closed, 22 cases of 4cm, 11 cases of 6cm, and 1 case of 10cm applicator. A 3 cm closed applicator was most often used on curved surfaces such as the nose. The cover of the closed applicator was used to flatten the curved surface for treatment.
The treatment field was controlled with a custom lead shield on the surface and a lead shield intranasally for septal mucosa protection. The radiation field size used was 1-2 cm larger than the lesion size. The field size margin was defined as the difference between the larger visible radius of the lesion and the larger radius of the field size.
Fractionation, as defined by single radiation dose, frequency, and total delivered dose, was based on the standard TDF (time dose fractionation) tables staying within 90-110 TDF for optimal biological efficacy.14 Most lesions treated (118 of 131) were between 98 and 102 TDF, while only 2 fell outside the 90-110 range. The frequency of treatment varied based on patient convenience and availability (Table 1). The mean fraction dose was 360 cGy (SD 76) delivered in an average of 13 fractions (SD 4) over a mean of 3 weeks (SD 1). The mean total dose was 4544 cGy (SD 377).
Table 1: Fractionation Scheme
|
Fractionation Dose Range (cGy) |
Number of Lesions |
Mean Fractionation Dose (cGy) |
Mean Fractions Total |
Mean Fractions delivered per week |
Mean Total Dose (cGy) |
|
260-290 |
13 |
269 |
19 |
5 |
5070 |
|
310-350 |
62 |
319 |
15 |
5 |
4735 |
|
368-400 |
15 |
379 |
12 |
3 |
4520 |
|
407-455 |
39 |
414 |
10 |
5 |
4109 |
|
647 |
2 |
647 |
6 |
2 |
3880 |
Frequency of treatment varied depending on patient availability. This table describes the fractionation scheme of the 131 total lesions treated, split up by dose range.
Results
Over a period of 7 years, 131 BCCs and SCCs were treated with SRT at our center. There was a 2:1 male to female ratio (90 male [69%], 41 female [31%]). The most common indication for SRT was basal cell carcinoma with 104 lesions (79%), followed by squamous cell carcinoma in 22 lesions (17%), and the remaining 5 being SCC in situ. The most common region was the head and neck area with 105 lesions (80%), and the most common anatomic structure was the lower nose (nasal tip and ala) with 60 lesions (46%). Table 2 lists the number of lesions for each location. Of 131 lesions, 117 were less than or equal to 2 cm and 14 were greater than 2 cm in lesion diameter. Overall mean radiation field margin added was 1.2 cm, with a median and mode of 1.1 cm and 1.0 cm, respectively. For lesions less than or equal to 2 cm, the mean field margin added was 1.1 cm. For lesions greater than 2 cm, mean field margin added was 1.6 cm.
Table 2: Number of lesions and patients organized by location
|
Location |
Number of Lesions |
Number of Patients |
|
Forehead/ Scalp |
22 |
20 |
|
Upper Nose* |
7 |
7 |
|
Lower Nose** |
60 |
56 |
|
Ear |
6 |
4 |
|
Cheek/Chin |
8 |
7 |
|
Neck |
2 |
2 |
|
Trunk*** |
8 |
5 |
|
Leg |
18 |
10 |
|
Total |
131 |
111 |
This table lists the number of lesions per location. *The upper nose includes the nasal dorsum and sidewall. **The lower nose includes the nasal tip and ala. ***The trunk includes the back, chest, and shoulder.
Most lesions (116) had not been previously treated. Three had prior curettage and desiccation, 5 had been previously excised, 8 had been treated with topical creams, and 4 had been frozen.
Follow-up time ranged from 2 weeks to 9 years. Of 131 lesions, 9 were excluded from recurrence analyses due to a short follow-up time of less than a year. Mean follow-up time was 5 years (SD 2), with a median and mode of 5 and 3 years, respectively. Of 122 lesions included in our analysis, the raw recurrence rate was 1.6% (2 lesions). One recurred at 2.5 years while the second recurred at 3.5 years. Mean time to recurrence was 3 years (SD 0.7). Of those that had 3 years or greater follow-up (94 cases), the recurrence rate was 2.1%.
The treatment frequently resulted in skin weeping and crusting, superficial minor bleeding, or superficial erosions that healed within 2 weeks after completion of treatment. More severe acute radiation ulcerations took up to 6 weeks to heal and occurred in 29 of 106 (27%) head and neck lesions, 5 of 8 (63%) trunk lesions, and 9 of 18 (50%) leg lesions. (Figure 1) (Table 3) A trend of higher rate of acute ulceration with higher fraction dose was observed with trunk lesions compared to head and neck lesions. Trunk lesions had a median dose fraction of 413 cGy (mean 402 cGy), while the head and neck lesions had a median dose fraction of 330 cGy (mean 359 cGy). As expected, higher dose fractions resulted in a higher rate of acute ulcerations.
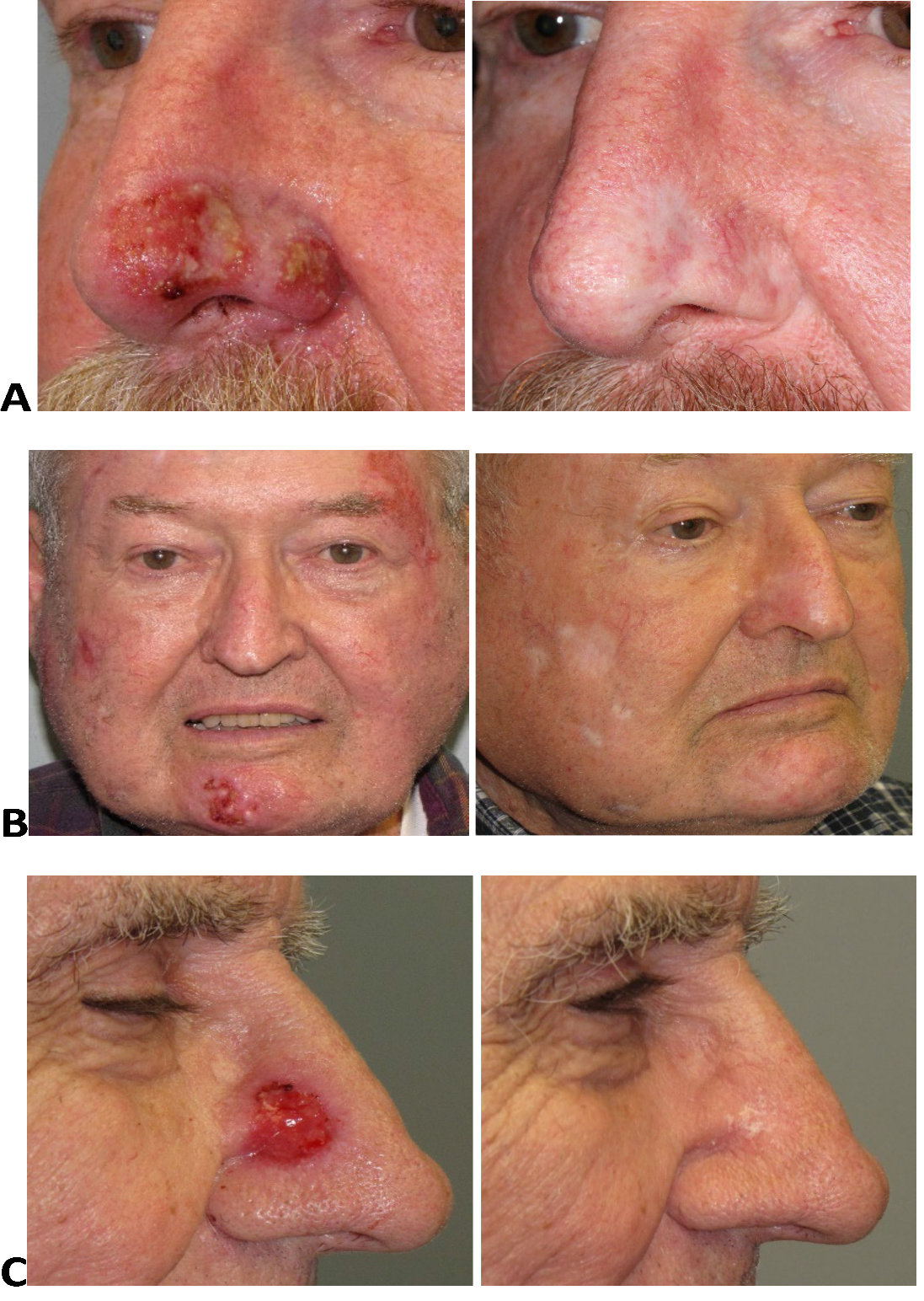
Figure 1: Consequences of Superficial Radiotherapy(A) Superficial ulceration 2 weeks after completion of a 3 week course of SRT (15 fractions), unusually severe hypopigmentation and skin atrophy 3 years after treatment.
(B) Superficial ulceration of the right cheek and chin 3 weeks after completion of a 3 week course of SRT (15 fractions), patchy hypopigmentation and skin atrophy 1 year after treatment.
(C) Superficial ulceration 2 weeks after completion of a 3-week course of SRT (15 fractions), flaky scaling of healed skin 4 weeks after completion of SRT.
Table 3: Acute and Delayed Ulceration Based on Location
|
Complications |
Acute Ulceration |
Delayed Ulceration |
|
Head and Neck |
29 (27%) |
0 (0%) |
|
Trunk |
5 (63%) |
0 (0% |
|
Leg |
9 (50%) |
5 (28%) |
Head and neck include upper nose, lower nose, forehead, scalp, cheek, chin, ear, and neck with a total of 106 cases. Trunk includes back, chest, and shoulder with a total of 8 cases. Legs included a total of 18 cases. Acute healing was defined as under 1 month. Delayed ulceration was defined as appearing after 1 month.
Delayed wound breakdown occurred in 5 of 18 leg lesions (28%) and appeared at a mean of 2.6 years after treatment (SD 2). (Table 3) These delayed radiation ulcers were treated with debridement, antibiotics, and wound care and took up to 5 months to heal.
Known and expected sequelae of SRT were predictable and included skin atrophy, hypopigmentation, telangiectasia, and loss of red vascular complexion. (Figure 1) Nasal skin showed less vascular skin tone change but demonstrated characteristic smoothing of sebaceous skin with thinning and atrophy. With nasal ala treatment, up to 2 mm of alar rim retraction was seen. Hair bearing scalp treatment areas always resulted in alopecia.
Discussion
Success of superficial radiation therapy for nonmelanoma skin cancers has historically been considered much lower than the success rate of surgery. Published reports place the recurrence-free success rate of SRT between 84% to 96% 9-12. Olschewski in 2006 set a new standard by reporting no recurrences after a highly fractionated course of superficial radiotherapy for BCC’s.15 Higher fractionation by using lower fraction dose of 300 cGy was delivered in 19 sessions over 4 weeks. The energies and, thereby, depth of treatment used were higher than most other reported studies (70-75 kVp). Additionally, 10-15 mm margins15 were wider than the 5-10 mm margins traditionally chosen in other studies. A median of 3 years’ follow-up of 104 treated lesions likely slightly underestimated the recurrence rate. Longer follow-up or larger patient numbers could have identified a recurrence. Including SCC’s in the analysis could have also affected the recurrence, as has been shown in other studies.
Silverman in 1992 found a much higher recurrence rate for BCC at 5 years (7.4%).16 However, soft X-ray machines with lower energies and depth of treatment were used (29-50 kVp). Higher doses of 680 cGy were delivered over 5 fractions in a hypofractionated schedule. At least 5 mm margins were used. Other studies had also shown higher recurrence rates with the use of soft X-rays (20-50 kVp) and hypofractionation.12,17,18
Finally, Cognetta in 2012, reported a large study of 1715 treated BCC’s and SCC’s yielding a 2.6% raw recurrence rate, 1.9% 2 year and a 5% 5-year recurrence rate.19 Like Olschewski, he used a higher energy X-ray machine (80kVp). Unlike Olschewski, he used a hypofractionated dosing with fractions up to 700cGy delivered over 5-7 fractions with tumor margins of 5-10 mm. The time interval for which these fractions were delivered was not explicitly stated.
In our study, both BCC and SCC were evaluated. No lips or eyelids were treated, and the most frequent location of treatment was the lower nose. We used energies between 50-95 kVp, and margins of 5-15 mm. Our fractions varied among patients but mostly remained under 400cGy. Our raw recurrence rate is 1.6% with a median 5-year follow-up.
Our finding of 1.6% recurrence rate is the only study known to us that supports Olschewski’s (2006) results. Evaluation of the literature reveals a pattern that is supported by our data that a low single digit recurrence rate is achievable with SRT under certain conditions. Higher number of fractions (lower fraction dose), wider margins (1 cm), and deeper treatment (50 kVp or greater) are the contributing factors. Tumor selection and its margin estimation play a significant role in the success of SRT as well.
One of our indications for SRT was multiple cancer management. We have treated 2 to 3 lesions in one course of radiotherapy. This was primarily for patient convenience. In fact, patient lifestyle and convenience were significant factors in choosing SRT and the fractionation scheme. Our center’s approach to skin cancer management is biased by our availability of advanced reconstructions of skin cancer defects. This diminished the consideration of post-reconstructive deformity as an indication for SRT.
Although used as an indication for radiation, anticoagulant therapy should not be a rationale for radiation treatment of skin cancers. Current literature disputes this as a significant risk of surgical skin cancer treatment.20-22
We have avoided eyelid treatment for two reasons. Treatment often requires a corneal shield which is uncomfortable to place and risks a corneal abrasion. There is a post-treatment risk of dryness and eye irritation. Lips were also not treated due to the complexity of dental shielding and its involvement of dental specialists.
Based on clinical observation, tumor thickness greater than 5 mm was also an exclusion criterion. An absolute contraindication to radiation in our center was full thickness cancer involvement over bone. The induction of hypoxia by radiation and necrosis of tumor creates a set up for cancer recurrence. The periphery of the ulcerated tumor has relative hypoxia having barely survived necrosis. This paired with underlying hypoxic exposed bone induces a hypometabolic cellular state of cancer cells leading to radiation resistance. Our contraindications include full thickness cancer of thin scalp skin over calvarium and thin medial canthal skin over bone. Full thickness cancer involvement of soft tissue triangle of the nasal tip may be another contraindication for SRT. The thin skin can suffer from the same hypoxic rim effect leading to recurrence. This may have been the cause of one of our reported recurrences. Further study on the effect of different fractionation schemes is needed to better understand nasal soft tissue triangle response.
SRT carries a cost of recovery for many patients. Acute erythema arrives at 2 weeks into a 3-week treatment and resolves within 2 weeks of completion. Dermatitis with crusting and oozing may begin near completion of a 3-week course and resolves within 2 weeks. Most patients experience erythema and crusting to some degree. Acute ulcerations are less common, especially in the head and neck, and resolve within 2-6 weeks with no lasting damage.
Lower legs have a uniquely difficult course of healing. Up to 50% of our leg lesions developed acute ulcerations. More significantly, 28% of lower legs developed delayed ulcerations that took up to 5 months to heal. All of them occurred in patients over 70 years of age. The foundational reason is the hypoxic devascularization of radiated areas. The triggering event of delayed ulceration was either spontaneous breakdown, minor trauma, or an acute onset of edema. Unless other fractionation schemes can achieve better outcomes, SRT should not be used in lower legs.
Radiation long term visual consequences are always present in various forms. Classic skin thinning, hypopigmentation, loss of pink complexion, and telangiectasia present months after treatment. (Figure 1) Nasal ala rim was observed to develop up to 2 mm of cephalic retraction in some patients. However, the nose and especially the lower third (tip and ala) uniquely tolerates radiation with less visible long-term changes. Even sebaceous skin of the nose has a favorable outcome with skin smoothing. Biopsy contour deformity, if present before, remains visible after SRT.
The patches of complexion changes of the face after SRT are a common occurrence that perhaps can be softened with serrated shields for blending of skin effect. (Figure 1B) This visual consequence should be contrasted to surgical repair scars or even secondary intention healing in choosing options for skin cancer treatment.
A limitation to our study is the variable fractionation schemes used among the population of patients with variable total doses, number of fractions, and fractional dose. Also, longer follow-up may have discovered additional recurrences.
Currently, Mohs surgery offers the highest published cure rates for non-melanoma skin cancer treatment - 99%.23 However, superficial radiotherapy (SRT) offers a notable treatment alternative to surgery for basal and squamous cell carcinomas. When patients and lesions are highly selected, adequate margins of treatment are chosen, hypofractionation is avoided, and higher energies are used, cure rates of SRT can approach that of Mohs surgery.
Conflict of Interest Statement: Dr. Madorsky has nothing to disclose. Ms. Meltzer has nothing to disclose. Dr. Miller has nothing to disclose.
Funding: Funding was received from the Skin Cancer and Reconstructive Surgery Foundation.
References
- Röntgen WC. Über eine neue Art von Strahlen. 1896, Berlin-Göttingen-Heidelberg, Springer.
- Williams, Francis Henry. The roentgen rays in medicine and surgery. The Macmillan Company, 1902.
- Coutard H. Roentgen therapy of epitheliomas of the tonsillar region, hypopharynx, and larynx from 1920 to 1926. AJR Am J Roentgenol. 1932; 28: 313.
- Coutard H. Principles of x-ray therapy of malignant diseases. Lancet. 1934; 2: 1-12.
- Goldschmidt H. Ionizing radiation therapy in dermatology. Arch Dermatol. 1975; 111(11): 1511-1517.
- Madorsky, Simon J. “History of Radiotherapy in Dermatology.” Skin Cancer and Reconstructive Surgery Center, 12 Dec. 2012, scarscenter.com/radiation-therapy/history-of-radiotherapy-in-dermatology/.
- Kingery FA. Radiation therapy in dermatologic training centers. J Am Acad Dermatol. 1986; 14: 1108-10.
- Thom GA, Heywood JM, Cassidy B, et al. Three-year retrospective review of superficial radiotherapy for skin conditions in a perth radiotherapy unit. Australas J Dermatol. 2003; 44: 174-179.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989; 15(3): 315-328.
- Leibovitch I, Huilhol SC, Selva D, et al. Basal cell carcinoma treated with mohs surgery in Australia II. Outcome at 5-year follow-up. J Am Acad Dermatol. 2005; 53(3): 452-457.
- Ashby MA, Smith J, Ainslie J, et al. Treatment of nonmelanoma skin cancer at a large australian center. Cancer. 1989; 63: 1863-1871.
- Zagrodnik B, Kempf W, Seifert B, et al. Superficial radiotherapy for patients with basal cell carcinoma. Cancer. 2003; 98(12): 2708-2714.
- BJR Suppl. 25 (1996) Central Axis Depth Dose Data for Use in Radiotherapy. British Journal of Radiology, Suppl, 25, London.
- Orton CG, Ellis F. A simplification in the use of the NSD concept in practical radiotherapy. Br J Radiol. 1973; 46: 529-537.
- Olschewski T, Bajor K, Lang B, et al. Radiotherapy of basal cell carcinoma of the face and head: importance of low dose per fraction on long-term outcome. JDDG. 2006; 4: 124-131.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. J Dermatol Surg Oncol. 1992; 18: 549-554.
- Barysch MJ, Eggmann N, Beyeler M, et al. Long-term recurrence rate of large and difficult to treat cutaneous squamous cell carcinoma after superficial radiotherapy. Dermatology. 2012; 224: 59-65.
- Strom T, Harrison LB. Radiotherapy for management of basal and squamous cell carcinoma. Curr Probl Cancer. 2015; 39: 237-247.
- Cognetta AB, Howard BM, Heaton HP, et al. Superficial x-ray in the treatment of basal and squamous cell carcinomas: a viable option in select patients. J Am Acad Dermatol. 2012; 67(6): 1235-1241.
- Kraft CT, Bellile E, Baker SR, et al. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015; 17(2): 103-107.
- Bordeaux JS, Martires KJ, Goldberg D, et al. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011; 65(3): 576-583.
- Chen A, Albertini JG, Bordeaux JS, et al. Evidence-based clinical practice guideline: Reconstruction after skin cancer resection. J Am Acad Dermatol. 2021 Apr 26: S0190-9622(21)00505-3. Epub ahead of print.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989; 15: 315-28.
