Minireview about Medicinal Copaiba Oil in the Treatment of Skin Diseases
Katieli da Silva Souza Campanholi1*, Ranulfo Combuca da Silva Junior 1, Gustavo Braga 1,3, Flávia Amanda Pedroso de Morais 1,2, Rodolfo Bento Balbinot2, Wilker Caetano 1
1Chemistry Department, State University of Maringa, Maringa, Parana, Brazil.
2Department of basic health sciences, State University of Maringa, Maringa, Parana, Brazil.
3Federal Institute of Education, Science and Technology of Parana, Telemaco Borba, Parana, Brazil.
Abstract
The therapeutic benefits of copaiba oil-resin have encouraged its use in developing dermatological emulsions. Here, the anti-inflammatory, healing, antimicrobial, analgesic, and permeation-promoting properties of this medicinal oil are shown by reviewing articles from 2005 to 2021. Their properties encourage research to develop an herbal medicinal effective in treating skin diseases and cutaneous leishmaniasis.
Introduction
The Brazilian scenario of infectious and parasitic diseases faced a significant increase from 2018, the so-called epidemiological transition. The battle between humanity and pandemics is a significant challenge for public health, especially for developing countries such as Brazil.1 As a side effect of the ecological mismatch, Brazil faces the proliferation of epidemic diseases, such as leishmaniasis. Figure 1 shows the distribution of confirmed tegumentary leishmaniasis cases in 2020, emphasizing the north Brazilian region. In recent years, the frequency of this parasitosis has raised medical and scientific concerns, requiring the search for effective and accessible strategies for the population served by the Unified Health System (SUS).
Figure 1: Confirmed cases of leishmaniasis separated by regions of Brazil. Data from 2020 was updated on 10 August 2021 in the DATASUS access platform. Information plotted by the author.
The higher incidence in the north Brazilian region is linked to the lack of basic sanitation, scarce health care, the precarious economic situation, disorderly urban occupation, excessive contact with animals that serve as reservoirs of the disease, and deforestation and mining activities.2,3
Leishmaniasis is transmitted by biting infected females (vectors, popularly known as the sand fly) of the Phlebotominae subfamily (Figure 2). The amastigotes (ingested by the fly during blood repast) convert to promastigotes form (flagellates) in the sand fly. Subsequently, the promastigotes form is inoculated into mammals' skin during biting.4
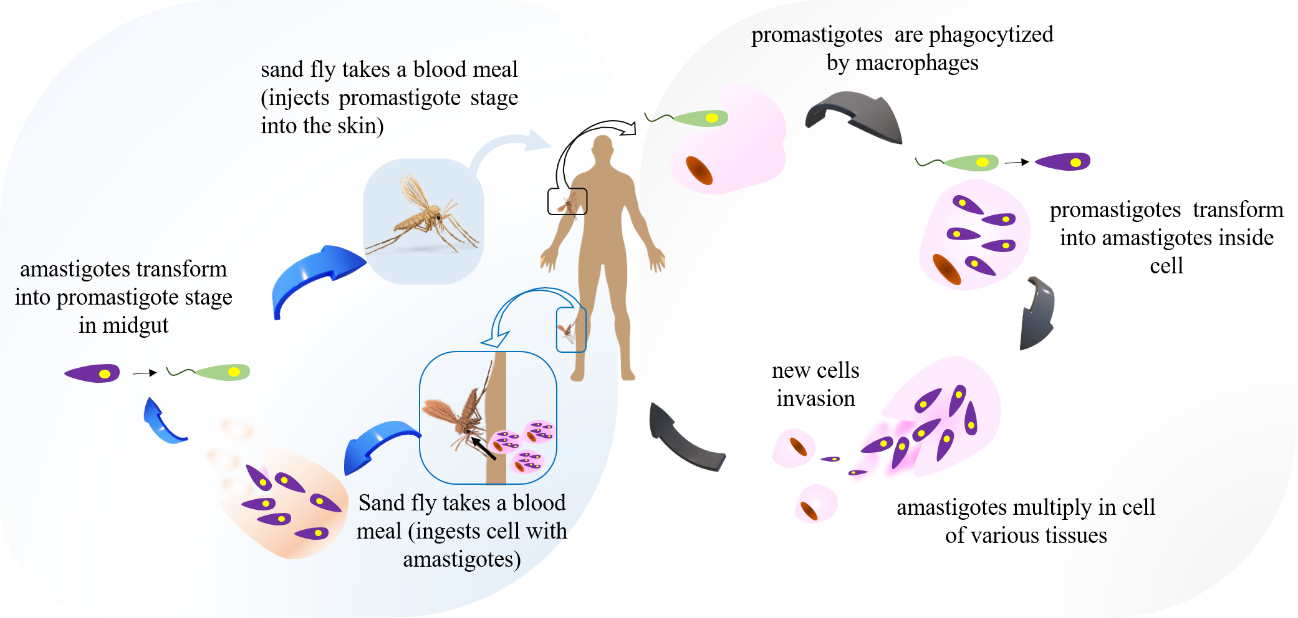
Figure 2: The life cycle of Leishmania species. Sand fly injects promastigotes during feeding. Promastigotes form are phagocytosed by macrophages and transform into amastigotes inside cell. Sand flies become infected by feeding on an infected host. Amastigotes convert to promastigotes form (infectious form for man) within the sand fly midgut.5
In intravenous or intramuscular doses, leishmaniasis treatment has been performed with N-methyl glucamine antimoniate and pentavalent antimonials. In addition, Amphotericin B deoxycholate or liposomal amphotericin B by intravenous infusion is also reported, with dosages established by the regulatory agencies.3 However, these therapeutic strategies have disadvantages due to dose-dependent acute adverse effects, which require hospitalization for drug administration, with the possibility of emergency tracheostomy.6
Dermatological treatments can be achieved using herbal formulations therapeutically based on copaiba oil-resin (COR).7–10 COR is a medicinal bioactive approved by the Food and Drug Administration (FDA) 11, 12 and commercialized in Brazil to combat more than 50 diseases, being included as a promisor agent against Sars-CoV-2 by molecular docking studies.13 This mini-review aims to bring to the scientific community the potential of COR for several skin diseases treatment, especially cutaneous leishmaniasis. This oil-resin has shown potential in leishmaniasis treatment, with IC50 values of 7.88 µg.mL-1 for promastigotes and 0.52 µg.mL-1 for amastigotes; data statistically equivalent (p>0.05) to Pentamidine and Amphotericin B.14
The main articles related to the benefits of using copaiba oil for dermatological purposes (dating from 2005 to 2021) were selected in this mini review. While COR has excellent potential in treating skin diseases, a small percentage of these articles address topical formulations with resin oil to treat cutaneous leishmaniasis.
Dermatological Therapeutic Effects of Copaiba Oil-resin
The interest in copaiba oil has increased significantly in recent years. For example, between 2005 and 2022, the Science Direct and Pubmed platforms provided 579 available searches with "copaifera topical" and "copaiba topical" as the selection, with more than 50% published between the years 2017 and 2022 (Figure 3). This fact demonstrates the growing recognition of the therapeutic effects of this oil-resin.
Figure 3: Articles available on the Science Direct and PubMed platform by selecting the terms "Copaifera topical" and "Copaiba topical".
The benefits of this medicinal oil can be attributed to its composition. Copaiba oil is rich in sesquiterpenes (volatile fraction) and diterpenes (resinous fraction).15 The antiinflammatory activity was attributed to hydrocarbons and sesquiterpenes (especially β-bisabolene and β-caryophyllene).7 Carvalho et al., (2005) evaluated the antiinflammatory and analgesic activity of Copaifera reticulata Ducke in topical administration (Rattus norvegicus). Besides, the authors evaluated granuloma tests related to the dermatitis inflammatory process. Daily doses of copaiba oil were from 517 mg to 1802 mg.kg-1 animal, being 1802 mg.kg-1 dose most effective in inhibiting edema and granuloma tissue.7
Estevão et al., (2013) evaluated the effects of copaiba oil ointment (Copaifera langsdorffii at 10 % w/w) on rat skin lesions. The authors found a less necrotic area and more granulation tissue, with bulky fibroblasts and collagen fibers arranged using copaiba ointment.16 Furthermore, De Oliveira et al., (2010) reported the advantages of using copaiba oil in dermatological treatments. The authors showed that the oil-resin acts as a skin penetration enhancer in drug combination therapy.17 This property is probably due to the emollient character of the formulation.18 Dermatology also highlights the use of copaiba oil in Acne Vulgaris and skin wound treatment, as reported in the literature.19, 20
Maragon et al., (2017) developed antimicrobial formulations composed of copaiba oils acquired from different regions, being OCA abbreviation regarding resin oil purchased from São Paulo, OCB from Belém, and OCC from Pará. The formulation matrix was chitosan/gelatin gels derived from pig skin in a 2:1 w/w ratio (QG matrix). Evaluations were performed against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Vero cell line cytotoxicity studies. The authors noted minimum inhibitory concentration (MIC) values for the QG matrix (without drug) between 31 and 62 μg.mL-1 for the three pathogens evaluated. The antimicrobial activities of OCA, OCB, and OCC copaiba oils (unformulated oil) showed the highest activity against S. aureus, with MICs of 2.0x103 μg.mL-1, 500 μg mL-1, and 62.5 μg.mL-1, respectively. Dealing with the QG emulsions containing copaiba oil, the authors reported improved results only for the QG-OCC system. A synergistic effect was observed for this formulation, reducing the bactericidal concentration compared to the individual components. Cytotoxicity evaluations of the individual components (OCA, OCB, and OCC) showed that only OCC showed no cytotoxic effect on Vero cells. However, after formulation, all systems were shown to be pharmacologically safe.21
Becker et al., (2019) studied the antinociceptive and antiinflammatory properties of a formulation of the copaiba oil (3 %) to treat sunburn-related inflammatory pain. The developed formulation was characterized by temporal stability, organoleptic characteristics, pH, spreadability, and rheological profiles. Furthermore, the authors used a UVB radiation-induced skin burn model (0.75 J.cm-2) in rats and performed daily administration of the cream formulation. Overall, the developed copaiba gel proved to be stable during the analyzed period and allowed antinociceptive and anti-inflammatory effects in animals exposed to UVB radiation. These benefits were linked to the presence of β-caryophyllene and other sesquiterpenes identified by gas chromatography. Therefore, the authors considered the use of copaiba oleoresin as a promising strategy.22
Nigro et al., (2020) evaluated the use of resin oil as a potential replacement for Coenzyme Q10 (CQ10). CQ10 is a beneficial substance in wound treatment due to its antioxidant and healing properties. However, when formulated, CQ10 offers low skin permeation capacity, in addition to its susceptibility to photodegradation. Its permeation is desired to protect keratinocytes from oxidative damage and favor fibroblast and collagen proliferation. The authors noted improvements in cell viability when using Q10 and copaiba oil-resin mixed formulation. Furthermore, the combination therapy showed benefits dependent on the droplet size of the emulsion; studies evidenced when comparing preparation methods involving manual and ultrasonic agitation.23
When it comes to wounds, some types present difficulties in healing and require new adjuvant therapies to be combined with conventional clinical treatments. In this process, micro-needling and low-frequency waves can increase the healing speed of deep wounds. Studies have already reported the advantages of the cutaneous administration of copaiba oil by micro-needling on collagen and fibroblast production.24 In the same way, the use of ultrasound associated with conventional therapy showed benefits. Patients with wounds followed up by the hospital in Ceará state, Brazil, were treated with 10 sessions of ultrasonic therapy associated with the gel with copaiba and tea tree oil. The authors found a more than 50% reduction in the initial lesion size by the 10th session. Some patients showed complete healing by the 10th session (2 patients of 14 evaluated).25
In patients with leishmaniasis, the immune status must be considered when choosing the therapeutic approach. Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine produced by macrophages that is important in defense of the host against infection by Leishmania species. However, the treatment of several diseases, such as rheumatoid arthritis and psoriasis, involves anti-TNF-α therapy. Consequently, these patients are usually afflicted with opportunistic infections, such as leishmaniasis and tuberculosis. Cases of cutaneous leishmaniasis in patients taking TNF-α are poorly described in the literature.26 The use of copaiba oil (Copaifera sp.) has also been suggested as alternative medicine in treating rheumatoid arthritis, with doses that are still under investigation in the scientific field. The main component of the oil-resin that shows anti-inflammatory activity is β-caryophyllene.27 There are indications that copaiba oil (100 µg/mL) also acts with mechanisms that inhibit TNF-α; however, this behavior is still being investigated.28
Antileishmanial Activity of the Copaiba Oil-resin
Although copaiba oil exhibits skin healing activities, its use as a leishmanicidal has been little explored. In Brazil, the works published by Santos et al., (2008) are considered a reference for making a broad sweep of copaiba oil species found in the Brazilian Amazon. The authors carried out important in vitro studies considering the antiprotozoal activity of the Copaifera multijuga, Copaifera officinalis, Copaifera reticulata Ducke, Copaifera lucens, Copaifera langsdorfii, Copaifera paupera, Copaifera martii and Copaifera cearenses against Leishmania amazonenses. The data showed different activity levels against the promastigote form of Leishmania amazonensis, with IC50 ranging from 5.0 to 22.0 g mL-1. The biological activities were attributed to the group of sesquiterpenes (especially β-caryophyllene, α-copaene, zingiberene, β-bisabolene, and bergamotene) and diterpenes (mainly hardwichiic, kovalenic, kaurenoic, polyallic, and copalic) existing in different amounts in the copaiba species. The Copaifera reticulata Ducke showed more significant activity than the others, with IC50 of 5.0 µg mL-1 for the promastigote form and 15.0 µg mL-1 for the amastigote form of Leishmania amazonensis after 72 h of incubation. Similarly, Copaifera reticulata Ducke showed marked activity against the amastigote form, with IC50 of 20.0 µg mL-1. The more significant activity of Copaifera reticulata Ducke than the others was attributed to the more significant presence of the component β-caryophyllene.29 The mechanism of action of β-caryophyllene is not well understood. However, leishmanicidal drugs target the glucose transport system and Leishmania-specific enzyme systems.14
Dhorm Pimentel de Moraes et al., (2018) developed nanoemulsions of copaiba and andiroba oils for testing L. infantum and L. amazonensis. The nanoemulsions were composed of water (10 mL), Tween 80 (0.4 g), Span-80 (0.4 g), and oil phase (1 g) obtaining NanoCopa (with copaiba oil) and NanoAndi (with andiroba) formulations. The systems showed no considerable coalescence effects over 90 days and maintained their sizes between 76 and 92 nm (polydispersity values near 0.15). The in vitro and in vivo results obtained by the authors were satisfactory. The promastigote form of both Leishmania species proved to be sensitive to NanoCopa and NanoAndi. Morphological analyses of the protozoa after nanoemulsions treatment induced an oval cell shape and retracted flagella. In addition, the authors found a reduction in the infection levels caused by L. infantum and L. amazonensis in macrophage cultures. The use of copaiba nanoemulsions proved beneficial when considering L. amazonensis-induced lesion size, parasite load, and histopathology profile in BALB/c mice. Similarly, the treatment of L. infantum infected animals reduced the parasite load in the spleen and liver and improved the histopathological profile.30
Stimulant-responsive Formulations for the Treatment of Cutaneous Leishmaniasis
The union of research groups at the State University of Maringá, Paraná State, Brazil, develops optimized formulations containing high copaiba oil content. Extensive studies are conducted to evaluate the maximum content of copaiba oil that can be incorporated into topical formulations while ensuring good mechanical and rheological properties. Although the benefits of the bioactive are known, little research has been done to generate a dermatological gel with high performance before and after administration. The work proposed by the University of Maringá certainly has high market potential.10,31,32 Figure 4 reports all the benefits of copaiba oil in dermatological treatments. Observations indeed resulted in the selection of the therapeutic oil in many Brazilian kinds of research.
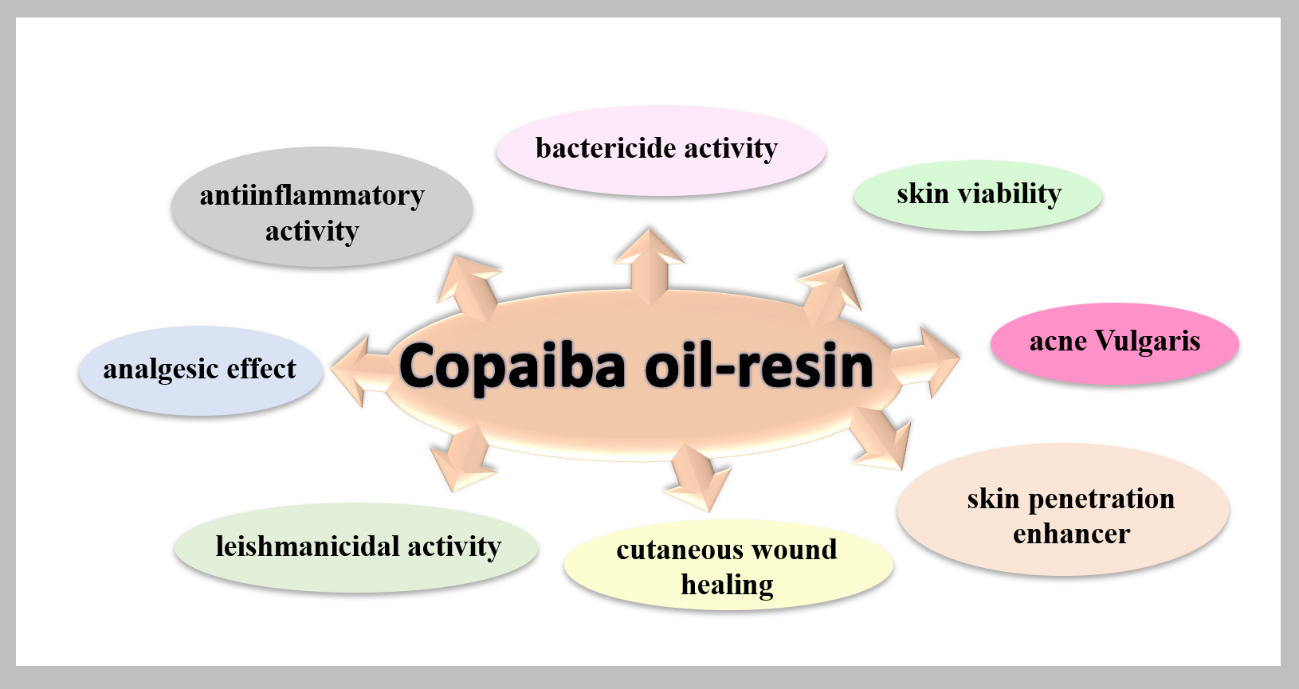
Figure 4: Activities reported in scientific articles for copaiba oil-resin.
Campanholi et al., (2021) sought an optimized thermoresponsive formulation containing high copaiba oil-resin. The authors proposed developing a dermatological gel to treat ulcerated skin or skin affected by cutaneous leishmaniasis. The evaluation of the stimulus-responsive properties took into account the range of viscosity values before and after the end of the gelling process. The authors worked with concentrations of copaiba oil ranging from 15-25 % w/w, Pluronic F127 from 18-22 % w/w, and bioadhesive polymer carbopol, from 0.2 to 0.3 % w/w. The mechanical and rheological characteristics showed losses when a high oil concentration was incorporated. At the upper planning limit for oil and Pluronic, the system showed high consistency and impaired stimulus-responsive properties. The best properties were seen in the systems with the lowest loadings of Pluronic oil. Thus, the authors suggested that the lower limit of the components is promising in further design and optimization studies.10 Figure 5 shows a photograph donated by the authors that shows the extensive study to improve the formulation with copaiba oil.
Figure 5: Image donated by Katieli S. S. Campanholi (photo) Ph.D. researcher, reporting on the cycle of new drug development performed at the State University of Maringa, Brazil.
Conclusion
The anti-inflammatory, leishmanicidal, healing, antimicrobial, analgesic, and permeation-promoting properties of copaiba stimulate studies seeking the development of high-performance drugs to treat cutaneous leishmaniasis.
References
- DataSUS. Leishmaniose Tegumentar Americana; 2021.
- Carmo RF, Luz ZMP da, Bevilacqua PD. Percepções Da População e de Profissionais de Saúde Sobre a Leishmaniose Visceral. Cien. Saude Colet. 2016, 21 (2), 621–628. https://doi.org/10.1590/1413-81232015212.10422015.
- Ministério da Saúde. Secretaria de Vigilância em Saúde. BRASIL. Manual de Vigilância Da Leishmaniose Tegumentar Americana, Departamen.; Ministério da Saúde, S. de V. em S., Ed.; 2007.
- Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet 2018, 392 (10151), 951–970. https://doi.org/10.1016/S0140-6736(18)31204-2.
- Esch KJ, Petersen CA. Transmission and Epidemiology of Zoonotic Protozoal Diseases of Companion Animals. Clin. Microbiol. Rev. 2013, 26 (1), 58–85. https://doi.org/10.1128/CMR.00067-12.
- Pelissari DM, Cechinel MP, Sousa-Gomes ML. de, et al., Tratamento Da Leishmaniose Visceral e Leishmaniose Tegumentar Americana No Brasil. Epidemiol. e Serviços Saúde 2011, 20 (1), 107–110. https://doi.org/10.5123/S1679-49742011000100012.
- Carvalho JCT, Cascon V, Possebon LS, et al., Topical Antiinflammatory and Analgesic Activities OfCopaifera Duckei Dwyer. Phyther. Res. 2005, 19 (11), 946–950. https://doi.org/10.1002/ptr.1762.
- dos Santos AO, Ueda-Nakamura T, Dias Filho BP, et al. Copaiba Oil: An Alternative to Development of New Drugs against Leishmaniasis. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–7. https://doi.org/10.1155/2012/898419.
- Valadas LAR, Gurgel MF, Mororó JM, et al. Dose-Response Evaluation of a Copaiba-Containing Varnish against Streptococcus Mutans in Vivo. Saudi Pharm. J. 2018. https://doi.org/10.1016/j.jsps.2018.12.004.
- Campanholi K. da S. S, da Silva JB, Batistela VR, et al. Design and Optimization of Stimuli-Responsive Emulsion-Filled Gel for Topical Delivery of Copaiba Oil-Resin. J. Pharm. Sci. 2021. https://doi.org/10.1016/j.xphs.2021.10.003.
- Símaro GV, Lemos M, Silva JJM da; et al. In Vivo Study of Anti-Inflammatory and Antinociceptive Activities of Copaifera Pubiflora Benth Oleoresin. Nat. Prod. Res. 2020, 0 (0), 1–7. https://doi.org/10.1080/14786419.2020.1855639.
- Alvarenga MOP, Bittencourt LO, Mendes PFS, et al. Safety and Effectiveness of Copaiba Oleoresin (C. Reticulata Ducke) on Inflammation and Tissue Repair of Oral Wounds in Rats. Int. J. Mol. Sci. 2020, 21 (10), 3568. https://doi.org/10.3390/ijms21103568.
- Santos WO, Carla F, Ferreira L. Molecular Docking Study of Copaíba Oil Interacting with the Spike Protein of Sars-CoV-2. Res. Sq. 2021, 0–14. https://doi.org/https://doi.org/10.21203/rs.3.rs-841081/v1.
- Rondon FCM, Bevilaqua CML, Accioly MP, et al. In Vitro Efficacy of Coriandrum Sativum, Lippia Sidoides and Copaifera Reticulata against Leishmania Chagasi. Rev. Bras. Parasitol. Veterinária 2012, 21 (3), 185–191. https://doi.org/10.1590/S1984-29612012000300002.
- Rodrigues Santana S, Bianchini-Pontuschka R, Bay Hurtado F, et al. Uso Medicinal Do Óleo de Copaíba (Copaifera Sp.) Por Pessoas Da Melhor Idade No Município de Presidente Médici, Rondônia, Brasil. Acta Agronómica 2014, 63 (4), 361–366. https://doi.org/10.15446/acag.v63n4.39111.
- Estevão LRM, Medeiros JP de, Baratella-Evêncio L, et al. Effects of the Topical Administration of Copaiba Oil Ointment (Copaifera Langsdorffii) in Skin Flaps Viability of Rats. Acta Cir. Bras. 2013, 28 (12), 863–869. https://doi.org/10.1590/S0102-86502013001200009.
- De Oliveira RVM, Ohara MT, Vila MMDC, et al. In Vitro Evaluation of Copaiba Oil as a Kojic Acid Skin Enhancer. Brazilian J. Pharm. Sci. 2010, 46 (2), 363–370. https://doi.org/10.1590/S1984-82502010000200024.
- Said dos Santos R, Bassi da Silva J, Rosseto HC, et al. Emulgels Containing Propolis and Curcumin: The Effect of Type of Vegetable Oil, Poly(Acrylic Acid) and Bioactive Agent on Physicochemical Stability, Mechanical and Rheological Properties. Gels 2021, 7 (3), 120. https://doi.org/10.3390/gels7030120.
- Da Silva AG, De Freitas Puziol P, Leitão RN, et al. Application of the Essential Oil from Copaiba (Copaifera Langsdorffii Desf.) for Acne Vulgaris: A Double-Blind, Placebo Controlled Clinical Trial. Altern. Med. Rev. 2012, 17 (1), 69–75.
- Martini CAN, Scapini JGS, Collaço LM, et al. Comparativa Dos Efeitos Do Óleo-Resina de Copaifera Multijuga e Da Nitrofurazona Na Cicatrização de Ferida Cutânea. Rev. Col. Bras. Cir. 2016, 43 (6), 445–451. https://doi.org/10.1590/0100-69912016006006.
- Marangon CA, Martins VCA, Leite PMF, et al. Chitosan/Gelatin/Copaiba Oil Emulsion Formulation and Its Potential on Controlling the Growth of Pathogenic Bacteria. Ind. Crops Prod. 2017, 99, 163–171. https://doi.org/10.1016/j.indcrop.2017.02.007.
- Becker G, Brusco I, Casoti R, et al. Copaiba Oleoresin Has Topical Antinociceptive Activity in a UVB Radiation-Induced Skin-Burn Model in Mice. J. Ethnopharmacol. 2020, 250, 112476. https://doi.org/10.1016/j.jep.2019.112476.
- Nigro F, Cerqueira C, Rossi A, et al. Development, Characterization and in Vitro Toxicity Evaluation of Nanoemulsion-Loaded Hydrogel Based on Copaiba Oil and Coenzyme Q10. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124132. https://doi.org/10.1016/j.colsurfa.2019.124132.
- Palheta C da SA, da Silva WMP, Couteiro RP, et al. Efeito Do Óleo de Copaíba Associado Ao Microagulhamento Na Pele de Ratos: Um Estudo Comparativo. Surg. Cosmet. Dermatology 2017, 9 (4), 290–295. https://doi.org/10.5935/scd1984-8773.2017941102.
- Mororó DGA, Brandão MGSA, Ponte VA, et al. Therapeutic Ultrasound Associated with Essential Oils of Copaiba and Tea Tree for Healing Skin Lesions. ESTIMA, Brazilian J. Enteros. Ther. 2020. https://doi.org/10.30886/estima.v18.942_IN.
- Gomes KWP, Benevides AN, Vieira FJF, et al. Leishmaniose Tegumentar Em Paciente Com Espondilite Anquilosante Utilizando Adalimumabe. Rev. Bras. Reumatol. 2012, 52 (3), 450–452. https://doi.org/10.1590/s0482-50042012000300014.
- Dini VSQ, Furtado S da C, Barcellos JFM, et al. Ação Anti-Inflamatória Do Óleo de Copaíba Em Artrite Induzida Em Modelo Animalâ¯: Uma Revisão Sistemática. Sci. Amaz. 2019, 8 (1), 1–12.
- Dias DS, Fontes LBA, Crotti AEM, et al. Copaiba Oil Suppresses Inflammatory Cytokines in Splenocytes of C57Bl/6 Mice Induced with Experimental Autoimmune Encephalomyelitis (EAE). Molecules 2014, 19 (8), 12814–12826. https://doi.org/10.3390/molecules190812814.
- Santos AO, Ueda-Nakamura T, Dias Filho BP, et al. Effect of Brazilian Copaiba Oils on Leishmania Amazonensis. J. Ethnopharmacol. 2008, 120 (2), 204–208. https://doi.org/10.1016/j.jep.2008.08.007.
- Dhorm Pimentel de Moraes AR, Tavares GD, Soares Rocha FJ, et al. Effects of Nanoemulsions Prepared with Essential Oils of Copaiba- and Andiroba against Leishmania Infantum and Leishmania Amazonensis Infections. Exp. Parasitol. 2018, 187, 12–21. https://doi.org/10.1016/j.exppara.2018.03.005.
- dos Santos AO, Costa MA, Ueda-Nakamura T, et al. Leishmania Amazonensis: Effects of Oral Treatment with Copaiba Oil in Mice. Exp. Parasitol. 2011, 129 (2), 145–151. https://doi.org/10.1016/j.exppara.2011.06.016.
- Santos AO dos, Ueda-Nakamura T, Dias Filho BP, et al. Antimicrobial Activity of Brazilian Copaiba Oils Obtained from Different Species of the Copaifera Genus. Mem. Inst. Oswaldo Cruz 2008, 103 (3), 277–281. https://doi.org/10.1590/S0074-02762008005000015.
