Beta-Glucans: A Biomimetic Approach for Reducing Chronicity in Delayed Wound Healing
Arthe Rajarajaran, Arivuoli Dakshanamoorthy*
Crystal Growth Centre, Anna University, Chennai
Abstract
The challenge which grows over time in the chronic wound healing is a self-care wound dressing. These wounds have unfavourable impact on patients wellbeing and also challenging to the health economy. The wound healing requires a complex series of physiological and immunological processes with adequate nutrition. Any derangement of immune signals at any stage can lead to impaired wound healing which alters the key transition point that lies between the inflammatory and proliferation phase that destroys the components of Extracellular matrix. The Extracellular matrix is responsible for regulating the growth factors and its receptors that are important for wound healing. To boost up the growth factor signalling and accelerating the chronic wound healing, a new biomimetic approach of mimicking the role of extracellular matrix helps in the development of instructive wound dressing. Thus this review deals in discussing the tremendous activity of the Natural polysaccharide called β-glucan on wound healing signalling which may help in mimicking the role of extracellular matrix.
Introduction
The thriving ubiquity of diabetes, obesity, aging population and change in life style continues to increase the frequency of the chronic wounds. Chronic skin wounds are one of the serious issue that is reaching epidemic proportions which are estimated to affect 20-60 million people worldwide by 20261. Unlike acute wounds, which heal after a certain period of time, chronic skin wounds heal slowly or not at all heal. These wounds can lead to long term hospitalization which is highest burden to the healthcare sector as there is a mortality of the patients as they have shown to cause loss of mobility and ability to perform daily tasks, limb amputation and poor quality of life. The effect of non-healing wounds on mortality has even been comparable to or worse than that of few common cancers like prostate, breast and colon cancer2.
Chronic wounds
The healing process starts from the hemostasis stage that is connected with forming a temporary matrix, secreting cytokines and other growth factors, and interaction of the latter ones with Extracellular Matrix(ECM), which initiates the repairing process, preparing the wound bed to the next stage of the healing process. In a healthy person with no underlying inhibitory factors an acute wound should heal within 3 weeks with the remodeling occurs over the next year or so. If a wound does not follow the normal path of healing, one of the phase of healing may be hindered and lengthened which makes the wound to becomes chronic3. Chronic wounds are thus defined as wounds, which have “failed to proceed through an orderly and timely process to produce anatomic and functional integrity, or proceeded through the repair process without establishing a sustained anatomic and functional result”4. Conventionally, a period of 6-8 weeks has been accepted by various authorities as the cut off time, beyond which the wound is labeled as chronic/non-healing3.
Chronic wounds and ECM breakdown
There are number of causes of delayed healing such as ischemia, wound infection, persistence of foreign body or bacterial proteins, chronic irritation, trauma and so on5. Ischemia is one of the key factor that makes the chronic wound to develop and makes it severe in the old age patients when it occurs repetitively. Ischemia decreases the blood supply to tissues leading to decreased oxygen and nutrients in the affected area causing inflammation in the tissues that triggers the cells to release factors such as chemokines, interleukins, leukotriences and complement system that attract neutrophils6. During the response against pathogens, neutrophils tend to release inflammatory cytokines and various other enzymes. Myeloperoxidase is one of the essential enzyme produced by neutrophils which inturn kills the bacteria by developing the reactive oxygen species (ROS)7. The increase in these enzymes and ROS production of neutrophils and other leukocytes damage cells that are essential for the proliferation phase by preventing proliferation and wound closure thereby causing damages in DNA, lipids, proteins, ECM and cytokines that normally aid the healing process8. Neutrophils remain extended in chronic wounds than that of acute wounds contributing to the elevated level of inflammatory cytokines and ROS. Also the wound fluid from chronic wounds has high amount of proteases and ROS which inhibit healing by inhibiting cell growth and breaking down growth factors and proteins in the ECM9.
ECM and therapeutic approaches for chronic wound healing
The therapeutic approaches observed based on the accurate knowledge about the pathophysiology of a chronic wound have broadly focused on developing methods to decrease the ECM degradation, restoration of a healthy ECM and production of artificial ECM to activate chronic wound healing process10. Thus development of biomimetic material is a favourable approach for chronic wound healing11. Every tissue inside the body has a unique set of cells and ECM proteins arranged into a distinctive architecture, thus requiring the properties of bioengineering materials to be designed in an organ-specific way12. The lengthed inflammation and higher level of Matrix Metalloproteniases (MMPs) at the wound site causes significant degradation of ECM that delays wound healing process leading to chronicity (Fig.1&2). Thus the development of therapeutic dressing to control and positively regulate MMPs balance helps in achieving faster healing. The design of biomaterial matrices has the challenge to mimic the function of ECM that helps in fibroblast migration at wound site13. Recently, natural polymers have highly attracted the scientific community interest. By knowing the biocompatibility and biodegradable, nature of the naturally occurring polymers helps in highest level of biomimicry, replicating the biological and physicochemical features of the native ECM. By looking into our natural surroundings and by re-using some of the discarded natural resources, several functional biomaterials can be easily identified and implemented for promising wound healing applications, with a reduced impact on the environment. Nature itself can be better inspiration to develop economical, reduced energy consumption and fully biodegradable materials, providing great environmental sustainability. The increase interest in the use of either protein-based or polysaccharide-derived dressings provides striking and reflects the growing approach of giving back what we borrowed from Nature14. Naturally derived polymers provide a versatile, multitasking and tunable platform to design appropriate extracellular environments that actively contrast the onset of infection and inflammations, while promoting tissue regeneration and scar remodeling.
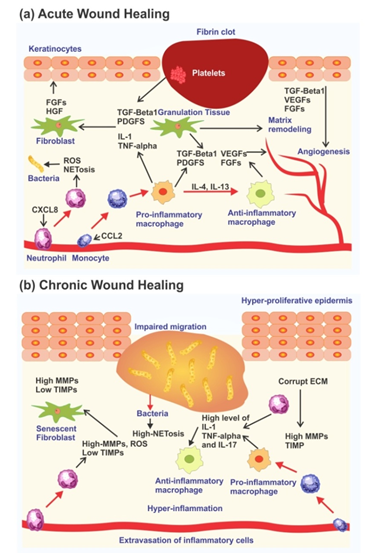
Figure 1: Acute and Chronic Wound Healing Process
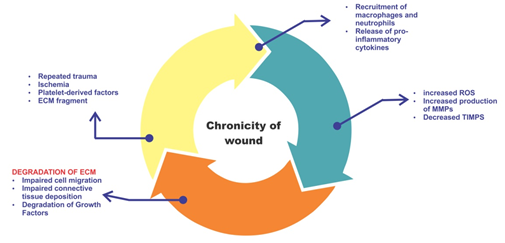
Figure 2: Inflammation cycle that contribute to the chronicity
Beta glucans as a naturally derived polymer
Natural polysaccharides are abundant in nature which are useful in many applications due to their unique properties. One of the most predominant class of polysaccharides is the β-glucans which are carbohydrate polymers that are found in the cell walls of many organisms such as bacteria, fungi, yeasts and some cereals like barley and oat15. All β-glucans comprises of glucose polymer linked by 1-3 linear glycosidic chain core of varing length and branching structures16. These branches that are derived from the glycosidic chain core are highly different with two main group of branching such as 1-4 or 1-6 glycosidic chains17. Also, different types of β-glucans exhibit distinct molecular weight, solubility and viscosity causing diverse physiological functions18. It is also most known for its powerful immune stimulant, antagonist of both benign and malignant tumors, anti-biotic properties and lower blood pressure or cholesterol levels19. Since beta-glucan enhances the production of growth factor that are essential for skin, promotes collagen biosynthesis and maintains skins moisture and elasticity20, we discussed the activity of various β-glucans on wound healing to make it evident for boosting the wound healing process of chronic wounds.
β-glucans also exhibited in vitro antimicrobial activity directly against a broad range of bacterial species, including E.coli, P.aeruginosa and S.aureus or indirectly by enhancing phagocytic activity and resistance towards the microbes21. In another study it was confirmed that the oat β-glucan showed antimicrobial activity against E.coli and B.subtilis22. β-glucan have a broad spectrum of effects on different cell types that can evident their proficiency on wound healing20. The ability of β-glucan to stimulate wound healing was first described by Leibovich and Danon in 198023, who observed faster re-epithilisation and increased macrophage activity and fewer polymorphonuclear neutrophils in the wound bed during inflammatory stage of repair. Various clinical trials reported that the topical application of fungal beta glucan accelerated healing in chronic ulcers24-28. β-glucan also activates macrophages that can remove cellular debris resulting from oxidative stress, thereby speeding up the recovery of damages tissue29. Fuste30 also confirmed that the barley β-glucan induced an early response in Human dermal fibroblast (HDF) cells favouring movement versus proliferation, and accelerated wound closure in vivo. According to Van den Berg31 et al., β-glucan have immuno-stimulatory capacity in temporary wound and show enhanced wound healing in burns. Curdlan enhanced migration, proliferation and wound closure of human kerotinocytes in a dectin-1 dependent manner both in vitro and in ex vivo. Numerous studies that are evident for the wound healing property of different type of β-glucan (Table 1).
Table 1. β-glucan types from various source and their respective wound healing activity
|
Name of the β-glucan |
Target cell type/ animal model |
Inference |
|
Baker’s yeast Glucan |
HDF |
increased the nuclear factor-1 binding capacity and enhanced collagen biosynthesis25 |
|
Porcine keratinocytes |
enhanced the keratinocyte proliferation26 |
|
|
3T3 fibroblast |
nanofibrous membranes enhanced the adhesion and proliferation of fibroblasts and kerotinocytes27 |
|
|
Venous ulcer biopsy |
enhanced the healing in venous ulcer28 |
|
|
Barley Glucan |
Adult Human dermal fibroblast (HDFa) / Mice |
induces an early response in HDF cell favouring movement versus proliferation30. |
|
Oat Glucan
|
Rats |
increased anti microbial activity, reduction of cholesterol and blood pressure22 |
|
Xyloglucan |
Wister rats |
exerted good healing effect in rats with severe wound32 |
|
NHEK, HaCaT and NHDF |
promoted skin regeneration33 |
|
|
Laminarin |
Human corneal epithelial cells |
enhanced the epithelial migration34 |
|
Paramylon |
Mice |
accompanied with a modest increase of inflammatory cytokines35 |
|
HEK 293 T cells |
acts as a bioactive supplement by boosting the cell proliferation capacity36 |
|
|
Curdlan |
Human Keratinocytes |
stimulated the cell proliferation and migration in a Dectin-1 dependent manner31 |
|
Swiss 3T3 fibroblast & wister rats |
Nanofibrous dressing of PVA/curdlan incorporated with Ag has fast healing of wound in rats37 |
|
|
β-(1,3–1,6)-D-Glucan from Aureobasidium pullulans
|
BALB / cnude mouse |
Membrane containing 50% β-glucan and Poly-(lactic co glycolic acid), accelerated the wound interactions38 |
|
ddY mouse |
Beta-glucan and chitosan complex enhanced the wound repair by activation macrophages and cytokine release39 |
|
|
Human dermal fibroblasts |
enhanced the dermal fibroblast migration and proliferation that modulated the effect of transforming growth factors40 |
|
|
Human dermal fibroblasts, adipose tissue derived stem cells |
boosted up the cellular response, migration and proliferation of both the cells41 |
|
|
Schizophyllan (SPG)
|
L292 Fibroblast |
SPG based nanofibrous scaffolds showed cell proliferation and cell migration42 |
|
|
PVA/SPG-AgNPs nanofibers showed anti-microbial activity thereby helping in reducing the infection in the wound43 |
|
|
Lichenan
|
NHEK and HaCaT keratinocytes |
stimulated human keratinocytes by specific mechanism into the terminal differentiation44 |
Conclusion
Wound healing is a repair and restoration of tissues through the series of stages that involves different cells and signalling molecules to regulate the cellular response and the dynamic remodelling of the extracellular matrix. Chronic wounds contain elevated levels of inflammatory cells, giving rise to more amount of proteases that degrades the ECM components, growth factors and receptors which are essential for wound healing. To restore and regulate the chronic wound healing cascade, a new approach of mimicking the extracellular matrix was discussed with a help of immuno modulatory polysaccharide called β-glucans. Various in vivo and in vitro studies discussed are evident to confirm the wound healing activity of beta-glucans from various sources. Also the β-glucans induces the proliferation and migration of keratinocytes and fibroblasts through speciï¬c receptors such as Dectin-1, CR3 or TLRs. These data also confirmed that β-glucans directly or indirectly modulate the activity of diverse cells and growth factors that are central to the reparative process. Thus β-glucans may interact with the innate immune system by regulating the macrophages and release the cytokine to produce growth factors/receptors thereby providing a temporary ECM for chronic wound healing.
Conflict of Interest statement
The authors declared that they have no conflicts of interest to this work.
References
- Lucilia P da Silva, Rui L Reis, Vitor M Correlo, et al. Hydrogel-based Strategies to advance therapies for chronic skin wounds. Annu Rev Biomed Eng. June 2019; 21: 145-69.
- Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes-related wounds and amputations worse than cancer?. Int Wound J. Dec 2007; 4(4): 286-287.
- Somprakas Basu, Vijay Shukla. Measurements in Wound Healing-Science and Practice. Complications of Wound Healing. London:Springer. 2012.
- Lazarus G, Cooper D, Knighton D, et al. Definitions and guidelines of assessment of wounds and evaluation of healing. Arch Dermatol. 1994; 130: 489-93.
- Jarbrink K, Ni G, Sonnergren, et al. Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Syst Rev. 2016; 5(152): 1-6.
- Birgit Kahle, Hans-Joachim Hermanns, Georg Gallenkemper. Evidence-based Treatment of Chronic Leg Ulcers. Dtsch Ärztebl Int. 2011; 108(14): 231-237.
- Tie- Shan Teng, Ai-ling Ji, Xin-Ying Ji, et al. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J Immunol Res. 2017; 1: 1-14.
- Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014; 20(7): 1126-1167.
- Schonfelder U, Abel M, Wiegand C, et al. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials. 2005; 26(33): 6664-73.
- Meilang Xue, Jackson CJ. Extracellular Matrix Reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015; 4(3): 119-136.
- Chantre CO, Hoerstrup SP, Parker KK. Engineering biomimetic and instructive materials for wound healing and regeneration.Curr Opin Biomed Eng. 2019; 10: 97-106.
- Bonnans C, Chou J, Werb Z. Remodelling the extracellulat matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014; 12: 786-801.
- Ramanathan G, Thyagarajan S, Sivagnanam UT. Accelerated wound healing and its promoting effects of biomimetic collagen matrices with siderophore loaded gelatin microspheres in tissue engineering. Mater. Sci. Eng. C. 2018; 93: 455-464.
- Suarato G, Bertorelli R, Athanassiou A. Borrowing from Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front Bioeng Biotechnol. 2018; 6: 1-11.
- Ahmad A, Anjum FM, Zahppr T, et al. Beta glucan : a valuable functional ingredient in foods. Crit Rev Food Sci Nutr. 2012; 52(3): 201-12.
- Aleem E. β-glucans and their applications in cancer therapy: focus on human studies. Anti-Cancer Agents Med Chem.2013; 13(5): 709-19.
- Kaur R, Sharma M, Ji D, et al. Structural features, modification and functionalities of beta-glucan. Fibers. 2020; 8(1): 1-29.
- Seo G, Hyun C, Choi S, et al.The wound healing effect of four types of beta-glucan. Appl Biol Chem. 2019; 62: 1-9.
- Bashir KMI, Choi JS. Clinical and Physiological Perspectives of β-glucans : The past, present and future. Int J Mol Sci. 2017; 18(9): 1-48.
- Majtan J, Jesenak M. β-Glucans: Multifunctional modulator of wound healing. Molecules. 2018; 23(4): 1-15.
- Chinnu K, Muthukumaran M, Mukund, et al. Antimicrobial and antifungal activity of isolated betaglucan from Chroococcus turgidus. Indian J Pharm Sci.2014; 4(4): 217-220.
- Shin MS, Lee S, Lee KY, et al. Structural and biological characterization of aminated-derivatized oat β-glucan. J Agric Food Chem. 2005; 53(14): 5554-5558.
- Leibovich SJ, Danon D. Promotion of wound repair in mice by application of glucan. J Reticuloendothel Soc.1980; 27(1): 1-11.
- Zykova SN, Balandina KA, Vorokhobina NV, et al. Macrophage stimulating agent soluble yeast β-1,3/1,6-glucan as a topical treatment of diabetic foot and leg ulcers: A randomized, double blind, placebo-controlled phase II study. J Diabetes Investig.Jul 2014; 5(4): 392-9.
- Wei D, Zhang L, Williams DL, et al. Glucan stimulates human dermal fibroblast collagen biosynthesis through a nuclear factor-1 dependent mechanism. Wound Repair Regen. 2002; 10(3): 161-168.
- Zulli F, Suter F, Biltz H, et al. Improving skin function with CM-glucan, a biological response modifier from yeast. Int J Cosmet Sci. 1998; 20(2): 79-86.
- Wu C, Chen T, Xin Y, et al. Nanofibrous asymmetric membranes self-organized from chemically heterogenous electrospun mats for skin tissue engineering. Biomed Mater. 2016; 11(3): 1-12.
- Medeiros ADV, Cordeiro SL, Cavalcanto JEC, et al. Effects of purified Saccharomyces cerevisiae (1→3)-β-Glucan on venous ulcer healing. Int J Mol Sci. 2012; 13(7): 8142-8158.
- Di Renzo L, Yefenof E, Klein E. The function of human NK cells is enhanced by beta-glucan, a ligand of CR3(CD11b/CD18). Eur J Immunol. 1991; 21(7): 1755-1758.
- Fuste NP, Guasch M, Guillen P, et al.Barley β-glucan accelerates wound healing by favoring migration versus proliferation of human dermal fibroblasts. Carbohydr Polym. 2019; 210: 389-398.
- Van den Berg LM, Zijlstra-Willems EM, Richters CD, et al. Dectin-1 activation induces proliferation and migration of human keratinocytes enhancing wound re-epithelialization. Cell Immunol. 2014; 289(1-2): 49-54.
- Hirose K, Sasatsu M, Toraishi T, et al. Novel Xyloglucan Sheet for the Treatment of deep wounds: Preparation, Physicochemical Characteristics, and in vivo healing effects. Biological and Pharmaceutical Bulletin. 2019; 42(8): 1409-1414.
- Nie W, Deters AM. Tamarind Seed Xyloglucans promote proliferation and migration of human skin cells through Internalization via stimulation of proproliferative signal transduction pathways. Dermatology Research and Practice.2013; 2013: 1-14.
- Choi JA, Oh TH, Choi JS, et al. Impact of β-1,3-glucan isolated from Euglena gracilis on corneal epithelial cell migration and on wound healing in a rat alkali burn model. Curr Eye Res. 2013; 38(12): 1207-13.
- Yasuda K, Ogushi M, Nakashima A, et al. Accelerated wound healing on the skin using a film dressing with β-glucan paramylom. In vivo. 2018; 32(4): 799-805.
- Arthe R, Arivuoli D, Ravi V. Preparation and characterization of bioactive silk fibroin/paramylon blend films for chronic wound healing.International Journal of Biological Macromolecules. 2019; Int J Biol Macromol. 2020 Jul 1; 154: 1324-1331.
- Basha RY, Kumar TSS, Selvaraj R, et al. Silver loaded Nanofibrous curdlan Mat for Diabetic Wound Healing: An in vitro and in vivo study. Macromol Mater Eng. 2018; 303(9): 1-15.
- Kim HL, Lee JH, Lee MH, et al. Evaluation of electrospun (1,3)-(1,6)-β-D-glucans/biodegradable polymer as artificial skin for full-thickness wound healing. Tissue Eng Part A. 2012; 18(21-22): 2315-22.
- Kofuji K, Yuzhou Huang, Tsubaki K, et al. Preparation and evaluation of a novel wound dressing sheet comprised of β-glucan-chitosan complex. React Funct Polym. 2010; 70(10): 784-789.
- Choi J, Kim JW, Jung G, et al. Effect of a β-glucan from Aureobasidium on TGF-β1-modulated in vitro dermal wound repair. J Toxicol Environ Health Sci. 2016; 8: 12-18.
- Woo YI, Park BJ, Kim HL, et al. The biological activities of (1,3)-(1,6)-beta-d-glucan and porous electrospun PLGA membranes containing beta-glucan in human dermal fibroblasts and adipose tissue-derived stem cells. Biomed Mater. 2010; 5(4): 1-8.
- Safaee-Ardakani MR, Hatamian-Zarmi A, Sadat SM, et al. Electrospun Schizophyllan/polyvinyl alcohol blend nanofibrous scaffold as potential wound healing. Int J Biol Macromol. 2019; 127: 27-38.
- Safaee-Ardakani MR, Hatamian-Zarmi A, Sadat SM, et al. Insitu preparation of PVA/Schizophyllan-AgNps Nanofiber as potential of wound healing: Characterization and cytotoxicity. Fibers Polym. 2019; 20: 2493-2502.
- Esch S, Gottesmann M, Hensel A. γ-Propoxy-Sulfo-Lichenan induces in vitro cell differentiation of human keratinocytes. Molecules.2019; 24(3): 1-11.
