Commentary: “Evaluating the Role of Small Particle Hyaluronic Acid Fillers Using Micro-droplet Technique in the Face, Neck and Hands: A Retrospective Chart Review”
Andreas Nikolis1,2*, Kaitlyn M. Enright2
1Associate professor of Plastic Surgery, Division of Plastic Surgery, McGill University, Montreal, Quebec, Canada
2Erevna Innovations Clinical Research Unit, Westmount, Quebec, Canada
Abstract
Hyaluronic acid (HA) substrates in facial rejuvenation have been a long-standing staple in the aesthetic injector’s offerings. The development of different technologies, variability in the concentration of HA within the gels and different types of cross-linking methodologies have led to the development of many skews across multiple companies. When addressing micro-droplet techniques whereby small aliquots of HA are deposited in the dermis, few have developed a safety and efficacy profile that supports claims of improved skin quality. The concept of adding HA into the dermis is inherently a correct one, as this glycosaminoglycan is able to bind and retain water in a significant fashion. Successful management of skin quality requires specific quantities of HA to be precisely placed at the appropriate depth using a reproducible volume. These aforementioned factors all contribute to successful skin quality improvements. We present a clinical summary of pearls and pitfalls in managing skin quality with micro-droplet HA that we have identified over the last 4 years.
It has been approximately three years since the author’s original article was published. Currently, the lead author wishes to share his pearls and pitfalls, learned from over four years of clinical experience using Restylane® SkinboostersTM Vital 20mg/ml and Vital Light 12mg/ml (Galderma, Uppsala, Sweden), herein referred to as SP-HAV and SP-HAVL, respectively.
Patient Selection
SP-HAV and SP-HAVL are an integral part of the aesthetic physician’s repertoire, as they can be used in almost all patients, both young and older alike. SP-HA is used in our clinic across generations of patients, beginning with millennials. The target group for SP-HAV and SP-HAVL are patients who could benefit from measurable improvements in skin health, especially those whose main concern is the appearance of dry or dehydrated skin. Therefore, the large majority of patients could benefit from such a treatment, as mostly everyone requires some sort of aid in maintaining skin quality. This is true regardless of lifestyle or skin care regimens. It is important to note that cosmetic creams claiming to be rich in HA generally have particles that are too large to penetrate skin pores and therefore lack significant efficacy. Only by depositing HA directly into the skin, as is the case with SP-HAV or SP-HAVL, can products exert their beneficial effects on skin quality in a reproducible fashion. Moreover, unlike other HA products, SP-HAV and SP-HAVL can be used in either thick or thin-skinned patients respectively, across all skin types I through VI of the Fitzpatrick’s Classification1; as long as the micro-aliquots are deposited into the deep dermis/subcutis junction thus avoiding visible and/or palpable surface irregularities. Although all patients will benefit from injections of HA micro-aliquots, those in more extreme weather conditions will benefit disproportionally more. Patients living in climates where the winters are long and environmental humidity is low, as well as patients in continuously warm and sunny climates require more frequent treatments to benefit their overall skin quality.
Choice of Product
An important concept for the novice injector as well as patients alike, to understand is that SP-HAV and SP-HAVL are not used as volumizers. SP-HAV and SP-HAVL are treatments to improve and maintain skin quality. This is their strength and is exactly why a clinician would select to use these products. While there may be indirect visible improvement of fine lines, this is secondary to the clinical improvement in skin health parameters. Patients wishing to improve such parameters including hydration, trans-epidermal water loss, elasticity, collagen and smoothness should consider the use of SP-HAV and SP-HAVL.2-6 Furthermore, using a non-animal stabilized hyaluronic acid (NASHA) based HA for micro-aliquot placement allows for targeted integration7 that is the product maintains its structural integrity over integrating or diffusing into the surrounding tissues.
Anatomical Guidelines
Although SP-HAV and SP-HAVL can be used anywhere on the face, the on-label indications in Canada SP-HAV is intended for the lower face (lower cheek, jawline) and dorsal hands, while SP-HAVL is intended for the lower cheek/jawline of the face and upper neck. Only SP-HAVL should be used in the neck, due to the degree of thinness of skin in this region. Furthermore, the whole neck should be treated as treatments focusing on visible horizontal folds or rhytids alone yield incomplete results. Again, when treating other regions of the face in an off-label fashion, injectors are urged to assess skin thickness vs choice of product. When in doubt, thin-skinned patients or regions should only be treated with SP-HAVL.
Technique
As previously mentioned, SP-HAV and SP-HAVL must be injected into the deep dermis/subcutis and uniformly deposited. If the product is placed at the proper depth, the chances of an unfavorable results are significantly minimized. Due to the non-linear contours of the face, the use of a cannula may make uniform product deposition difficult. For this reason, the senior author prefers using a sharp needle over a straight cannula to improve and maintain treatment outcomes in the face and neck. Needles should be changed frequently, as they become blunt with multiple punctures. A minimum of one new needle per side is recommended. Ice should be maintained on the contralateral side when not actively being treated. Dorsal hand injections require icing prior and immediately following treatment. Product can be placed at the same depth, but both techniques may be used (needle and cannula). What is important with hand treatments is a vigorous massage following injection as the product must be placed across the whole dorsum while avoiding veins, tendons, and the tendon sheaths surrounding the extensors. As with any injection, joint spaces should be avoided by remaining a minimum of one cm from the wrist and metacarpophalangeal joints.
When injecting, spacing of each aliquot of product can be between a few millimeters and up to 1 centimeter. However, we found that in patients with more visible skin damage, closer spacing is necessary. In areas of deeper lines, the distance can also be shortened. Although SP-HAV and SP-HAVL are not treatments for superficial rhytides, nor do we chase lines with the use of SB, the by-product of improving skin health parameters is a decrease in the depth and intensity of superficial rhytides. The SmartClick™ system (Figure 1) used with SP-HAV and SP-HAVL is novel and allows the injector to consistently place the same amount (0.01ml) of product in a reproducible fashion. Some injectors remove the SmartClick™ system when injecting SP-HAV or SP-HAVL. We recommend its continued use as it allows for complete control in quantity of HA placed. Typically, with aesthetic injections there are four treatment variables to consider, including the spacing and depth of product placement, frequency of treatments and volume per unit surface area. The SmartClick™ system removes the variable of inconsistent injection volume.
Figure 1: SmartclickTM System
Following treatment, it is essential to ensure that the aliquots are not visible or palpable. This can be accomplished by running an index finger over the injection sites, using a thin gel or cream (e.g., arnica or ultrasound gel). If the masses are palpable, massage each area accordingly. Most clinical trials employ a treatment regimen consisting of three sessions, every six weeks followed by a booster treatment every eight-to-twelve months. In clinical practice, we typically follow this treatment regimen, except in patients with significant skin quality issues, where the interval between treatments can be reduced to four weeks. We also prefer treating patients at four-week intervals during the winter months. This reduction in the interval time between treatments is supported by our own ultrasound investigations, which have demonstrated NASHA targeted tissue integration is maintained over time SP-HAV and SP-HAVL at 3-4 weeks following injections (data on file, Figure 2).
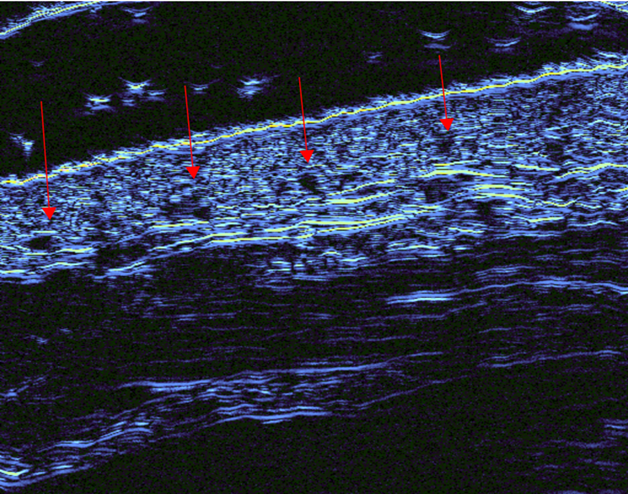
Figure 2: Three weeks following HA-SB injection. Note the continued presence of NASHA-SB with targeted integration. Red arrows demonstrate HA micro-aliquot placement in the deep dermis.
Novice injectors often inquire about the use of anesthetic with SP-HAV and SP-HAVL injections. While topical amide-type local anesthetics (e.g., Emla cream) can be used, we prefer icing the areas for five-to-ten minutes before the procedure. In addition to its anesthetic effects, it has the secondary benefit of constricting superficial vessels and reducing bruising and bleeding.
Managing Adverse Events
As with any injection, skin cleansing with an antiseptic topical is mandatory. During the treatment session, managing patient comfort with topical ice before, during and immediately after the treatment is paramount. Careful technique avoids superficial placement of HA. Furthermore, the system allows for a maximal injection volume of 0.01ml thereby controlling the volume of HA injected, while the depth of injection prevents placement of product in a named vessel. Consistently using the SmartClick™ system avoids variability in the quantity of product placed. Following treatment, during the initial follow-up visit, bruising should be re-evaluated as per the respective clinic’s protocol. If needed, pulsed dye laser can be used to help improve bruising. Given the large number of punctures required with SP-HAV and SP-HAVL in the face, neck and hands, it is important to avoid treating patients before any major event (e.g., wedding, vacation, photoshoot), as the likelihood of bruising is increased with the large amount of punctures and regions treated. In our experience, approximately 5-10% of treated patients will bruise with either SP-HAV and SP-HAVL. The number of regions bruised may vary, but with proper preconditioning of the tissues with ice, when present, there are few regions of pinpoint bruising. Lastly, managing patient expectations with SP-HAV and SP-HAVL is of utmost importance. Clinicians should have a thorough discussion with patients to ensure they understand that SP-HAV and SP-HAVL are not tissue volumizers but rather, treatments for improving skin quality and possible improvements in small rhytids (Figure 3). Concomitantly, SP-HAV and SP-HAVL can be used along with the patients’ normal skin care regimen.
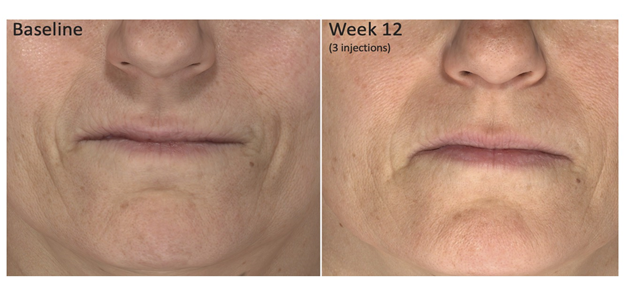
Figure 3: Baseline and twelve weeks following the commencement of HA-SB injections.
Conflict of Interest Statement
Dr. Andreas Nikolis is a consultant speaker and research collaborator for Allergan, Galderma and Merz Pharma.
References
- Fitzpatrick TB: The validity and practicality of sun reactive skin type I through VI. Arch Dermatol. 1988; 124: 869.
- Streker M, Reuther T, Krueger N, et al. Stabilized hyaluronic acid-based gel of non-animal origin for skin rejuvenation: face hand and décolletage. J Drugs Dermatol. 2013; 12: 990–994.
- Nikolis A, Enright KM. Evaluating the role of small particle hyaluronic acid fillers using micro-droplet technique in the face, neck and hands: a retrospective chart review. Clinical cosmetic and investigational dermatology. 2018; 11: 467-475.
- Bertucci, V, Lynde CB. Current Concepts in the Use of Small-Particle Hyaluronic Acid. Plast Reconstr Surg. 2015; 136(5 Suppl): S132–S138.
- Gubanova EI, Starovatova PA, Rodina MY. 12-month effects of stabilized hyaluronic acid gel compared with saline for rejuvenation of aging hands. J Drugs Dermatol. 2015; 14(3): 288–295.
- Kerscher M, Bayrhammer J, Reuther T. Rejuvenating influence of a stabilized hyaluronic acid-based gel of nonanimal origin on facial skin aging. Dermatol Surg. 2008; 34(5): 720–726.
- Lundgren, Björn, Sandkvist, et al. Using a New Photo Scale to Compare Product Integration of Different Hyaluronan-Based Fillers After Injection in Human Ex Vivo Skin. Journal of drugs in dermatology: JDD. 2018; 17: 982-986.
