Review of Immune Checkpoint Inhibitors and Radiotherapy Related Skin Toxicities
Margaret A. Kaszycki1*, Jonathan Leventhal2
1Frank H. Netter MD School of Medicine
2Yale School of Medicine
Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy, and their use in combination with radiation therapy (RT) has become increasingly utilized to optimize positive outcomes. The cutaneous adverse reactions from RT as well as ICIs are both well documented; however, in combination these cutaneous toxicities can be exacerbated. ICIs and RT may work synergistically to create an enhanced immune response against the tumor cells. This synergistic effect has been reported to occur both locally at the site of RT, as well as systemically via an abscopal effect. Fortunately, this combination of treatment does not increase the incidence of cutaneous reactions, although several cases have reported enhanced skin toxicity at the site of RT. RT is thought to create an ‘immunocompromised skin district’ or localized immune dysregulation in irradiated skin. This review summarizes previously published case reports and discusses the cutaneous adverse reactions from ICI and RT combination therapy. Properly identifying ICI and RT induced skin reactions depends on several factors including patient history, sequence of therapies, timing of reaction, and histological findings. Skin reactions from combination therapy can range in severity and include ICI-induced radiation recall dermatitis, as well as uncommon presentations of Stevens-Johnson syndrome, lichen planus, and bullous pemphigoid which are localized to or enhanced within areas of prior radiation exposure. It is important for oncologists and dermatologists alike to be aware of the spectrum of reactions associated with ICI and RT.
Introduction
The introduction of the first immune checkpoint inhibitor (ICI) was a breakthrough for immuno-oncology. Numerous clinical trials on ICIs and different combinations of ICIs have been approved and used for a variety of cancers. Although ICIs can target a range of hematological and solid tumor malignancies, melanoma, and non-small cell lung cancer (NSCLC) are amongst the most commonly treated malignancies. The overall survival, progression-free survival, and objective response rates of melanoma and NSCLC have improved due to PD-1 receptor inhibitors, such as nivolumab and pembrolizumab, and cytotoxic T-lymphocyte antigen-4 (CTLA-4) inhibitors, such as ipilimumab.1,2 Other PD-L1 inhibitors such as atezolizumab, avelumab, and durvalumab have also shown efficacy in a range of cancers including NSCLC and Merkel cell carcinoma.3 The mechanism behind these positive responses is through ICIs enhancing tumor immunity and promoting the activation and proliferation of effector T cells to recognize and destroy the cancer cells.1,3 PD-1 and PD-L1 inhibitors inhibit the programmed cell death protein 1 (PD1) and programmed cell death protein 1 ligand (PDL1) interaction. PD-1 on cytotoxic T-cells binds to PD-L1 expressed on tumor cells resulting in deactivation of the T-cell allowing tumor growth.4 CTLA-4 inhibitors block the CTLA-4 (CD152) receptor that is constitutively expressed on Tregs and is responsible for weakening immune responses and especially is known to diminish immune responses against infections and tumor cells.5 Due to PD-1 and CTLA-4 inhibitor's different mechanisms of actions, a combination of these treatments have even further prolonged survival rates in metastatic melanoma patients than compared to monotherapy alone.6
Radiation therapy (RT) is increasingly being utilized with ICI treatment for a variety of cancer treatments including brain metastases, NSCLC, melanoma, colorectal, breast, kidney, and prostate cancers. Advances in technology have allowed radiation oncologists to improve radiation delivery and effectiveness in controlling metastatic disease, as well as nodal basins suspected of harboring disease.7 Directed radiotherapy has been shown to improve the efficacy of ICIs in metastatic cancers through the abscopal effect. The abscopal effect is a regression of a distant non-irradiated metastatic tumor lesion from the effects of irradiation at the primary site. This effect has been observed in several metastatic melanoma cases and shows no dependence on the sequence of RT and ICI.8 The mechanism behind this is due to the ability of RT to re-program the tumor micro-environment to enhance T cell response and efficacy of tumor immunotherapy. RT causes apoptosis and necrosis of cancer cells causing a sufficient release of antigens from cancer cells, thus sensitizing, and priming the tumor cells to be killed by the host’s cytotoxic T lymphocytes (CTLs).9 RT also indirectly enhances MHC class I expression in a dose-dependent manner, thus increasing expression of antigens and subsequently activating CTLs. Once CTLs are activated, they can recognize and attack distant tumors, resulting in an abscopal effect.10
The addition of RT in patients with metastatic cancers is normally indicated for those with high risks of recurrence, recurrent, or unresectable tumors.8 RT plus ICI work synergistically by taking advantage of the different mechanisms of action, rather than simply adding two different methods together. The mechanism behind this is due to the ability of RT to re-program the tumor micro-environment in several ways to enhance T cell response and efficacy of tumor immunotherapy.11 A clinical trial by Victor et. al demonstrated that RT plus ICIs increase the CD8+/Treg ratio, induce MHC-I expression, increase the efficacy of ICIs, and result in abscopal responses.12 The use of ICIs plus RT may result in positive clinical outcomes and is generally safe if used cautiously.13,14 Neither the local effect of RT nor the survival benefit of ICI therapy are compromised when used in combination.138 Multi-site RT with concurrent ICI therapy is also safe and has positive clinical outcomes.9 Therefore, using ICI with RT results in enhanced direct killing of tumor cells and can be beneficial when used.
The use of ICIs is associated with a range of immune-related adverse events (irAEs) in multiple organ systems. Colitis, hepatitis, hypophysitis, and thyroiditis are examples of irAEs; however, the most common site of involvement is the skin, occurring in up to 50% of patients. Some cutaneous reactions require immediate attention and can interfere with treatment. Other cutaneous irAEs that do not usually interfere with treatment include vitiligo, pruritus, exanthems, and lichenoid reactions.15,16 More severe cutaneous irAEs include bullous pemphigoid, acute generalized exanthematous pustulosis, drug-induced hypersensitivity syndrome, and Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis.16 Due to the diversity of cutaneous reactions and the varied impact on quality of life and oncologic therapy, a multidisciplinary team should include a dermatologist in severe or recalcitrant presentations.
Similarly, as seen with ICIs, RT results in various skin reactions, most notably acute radiation dermatitis (RD) which occurs in a majority of patients. Identified risk factors include the presence of skin folds, higher BMI, current smoker, radiation-site, and radiation dose (gray (Gy)).17,18 Patients undergoing radiation treatment for head and neck cancer, breast cancer, and lung cancers are most likely to experience radiation dermatitis because of the higher required radiation doses.19
Post-radiation care with gentle washing with a mild soap can help reduce erythema at the radiation site. Acute RD following cumulative radiation doses of less than 20 Gy usually presents as generalized erythema, dryness, hair loss, and hyperpigmentation. At doses between 20-40 Gy pruritus and dry desquamation can occur. At doses greater than 40 Gy, moist desquamation may occur which often necessitates interruption of RT to allow for re-epithelization.18 Chronic skin changes may occur months to years after RT including epidermal thinning, dermal atrophy, vascular injury, telangiectasias, induration, pigmentary alteration, and fibrosis or thickening of the dermis.20,21 In addition, radiation recall dermatitis (RRD) may occur months to years after RT during treatment with anti-neoplastic drugs, whereas radiation enhancement may develop during concomitant medical therapy.22,23
Skin toxicities have been well reported for both ICIs and RT individually. This review aims to summarize the reported cutaneous toxicities during combination therapy and highlight potentially enhanced cutaneous adverse effects from ICI and RT. A previous meta-analysis by Yan et al.24 demonstrated no increased incidence of cutaneous adverse reactions from ICI plus RT; however, to the best of our knowledge, this is the first systematic review and comprehensive summary of published case reports on skin toxicities from ICI and RT.
Methods of Literature Search
Figure 1 shows the flow diagram of our systematic literature search and screening. A literature search was performed in PubMed and Scopus using the following search criteria: “Radiotherapy OR radiation therapy” AND “Checkpoint inhibitor OR ipilimumab OR nivolumab OR pembrolizumab OR atezolizumab OR avelumab OR cemiplimab OR durvalumab OR programmed cell death OR programmed death-ligand OR PD-1 OR PD-L1 OR CTLA-4” AND “skin OR rash OR cutaneous OR dermatitis”. The search was performed with no restrictions on date or language and was limited primarily to case reports. Of the 219 articles retrieved in the search, 14 were selected. An additional four articles identified from citations were added, totaling 19 papers and 21 cases included in the review.23,25-32 Inclusion criteria focused on including articles which primarily reported on skin toxicities from combination ICI and RT. Articles that were excluded either did not report cutaneous reactions or the cutaneous reactions did not occur from combination therapy.
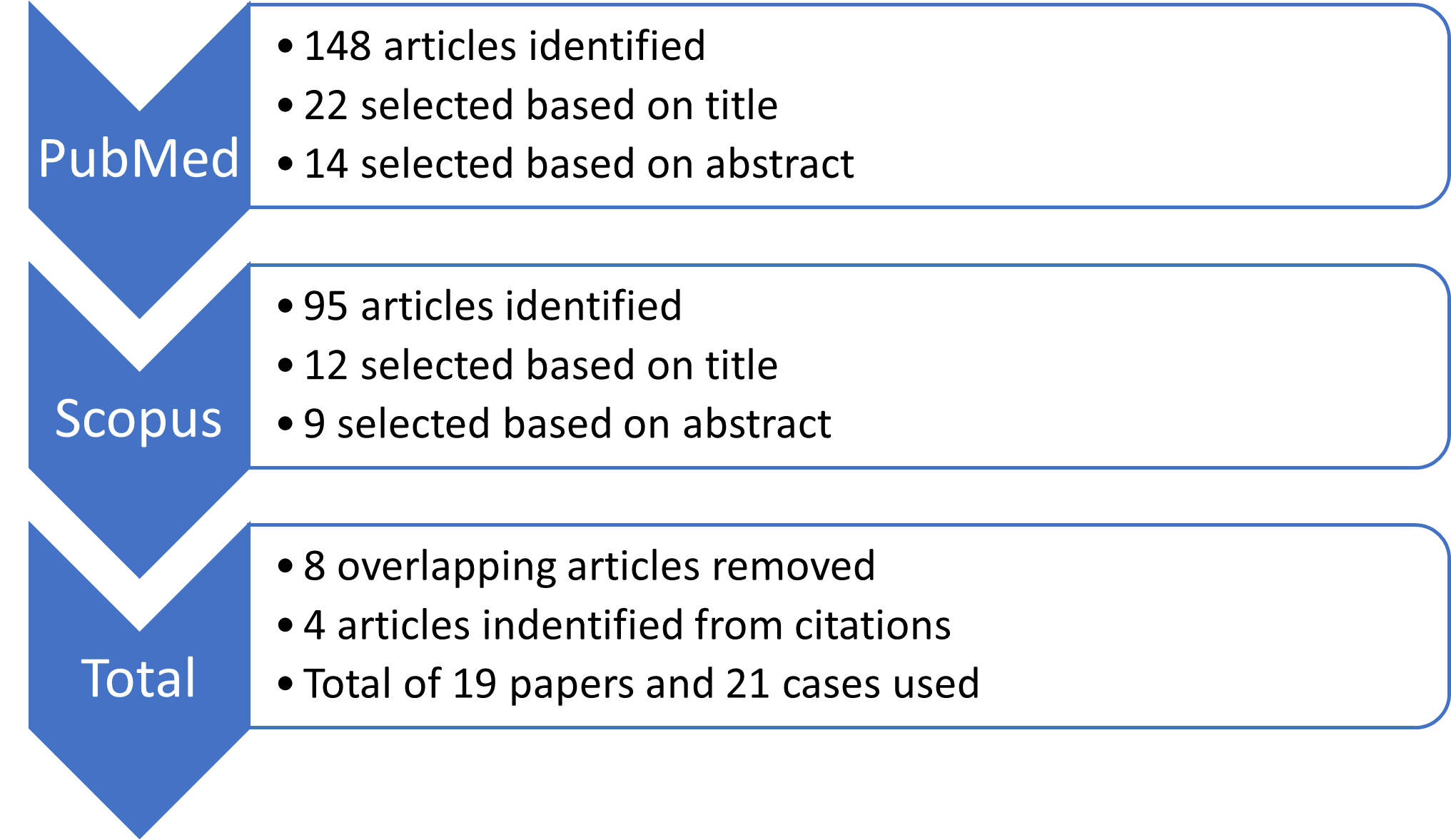
Figure 1: Flow diagram of literature search.
Results
A total of 21 reported cases of skin toxicities associated with ICI and RT were reviewed (Table 1). The median age was 62, ranging from ‘20s to 76 years old with eight females, eight males, and 4 unreported genders. Of the cancers being treated, 33% (n=7) accounted for melanoma, 24% (n=5) lung cancer, 19% (n=4) breast cancer, 19% (n=4) squamous cell carcinoma, and 4% (n=1) renal cell carcinoma. The most frequently reported skin toxicity was ICI-induced radiation recall dermatitis (n=10), followed by SJS (n=4), lichen planus (n=3), moist desquamation (n=2), maculopapular rash with RD (n=1), and bullous pemphigoid (n=1). Of note, one patient experienced lichen planus associated with ICI plus RT on two different occasions and thus was counted as two individual cases.25,26
Table 1:Skin toxicities associated with radiation and ICI therapy case reports organized by sequence of therapy
d/c: discontinued; fx: fractions; Gy: Gray; ICI: Immune checkpoint inhibitor; N/a: not available; RD: radiation dermatitis RRD: radiation recall dermatitis; RT: radiation therapy; SCC: Squamous cell carcinoma; SJS: Stevens-Johnson syndrome; SCLC: Small cell lung cancer
|
ICI therapy started following completion of RT |
||||||||||||
|
Study |
Age, Gender |
Cancer, stage, time of diagnosis |
Radiation site, dose (Gy), treatment length |
ICI, dose, treatment length, cycles |
Previous therapy |
Time interval between RT and skin reaction |
Time interval between ICI and skin reaction |
Skin reaction |
Management |
Histology |
||
|
Shah27
|
63, Male |
SCC of uvula and soft palate, stage IV
Time of diagnosis: N/a
|
Oropharynx and bilateral neck
Dose and treatment length: N/a
|
Nivolumab (anti-PD1)
Dose: N/a Cycles: 1 |
No |
8 days from last RT |
7 days after 1st dose |
SJS accentuated in radiation field * |
ICI d/c. Supportive care. |
Pauci-inflammatory interface process with numerous dyskeratotic cells, subepidermal split and areas of full thickness epidermal necrosis |
||
|
Nakashima28 |
61, Male
|
SCLC, Stage IIIc
Time of diagnosis: N/a |
Mediastinal lymph nodes and pericardium
Dose: 30/3 fx
Treatment length: N/a |
Atezolizumab (anti-PDL1)
Dose: 1200mg Cycles: 1 |
Carboplatin and nab-paclitaxel |
>21 days from last RT |
21 days after 1st dose |
Grade 3 ICI induced RRD * |
IV methyl-prednisolone and oral prednisolone. Improvement in 2 weeks. |
Interface dermatitis with perivascular lymphocytic inflammatory cell infiltration. |
||
|
Rouyer 29
|
61, Male |
Pulmonary epidermoid carcinoma
Stage and time of diagnosis: N/a |
Mediastinum: 50
Left hilum: dose: 60
Surgical area: 66
L knee: 30
Lumbar vertebrae: 20
Total dose: 226Gy
Treatment length: 1 year |
Nivolumab (anti-PD1)
Dose: N/a Treatment length: 2 weeks Cycles: 2 |
No |
30 days from last RT |
5 and 14 days after 2nd ICI dose a |
SJS accentuated in radiation field * |
N/a |
focal vacuolization of the basal layer and junctional detachment, margination of lymphocytes and some keratinocyte necrosis, suggesting SJS |
||
|
Dhanushkodi30 |
34, Female |
Triple negative breast cancer
RT treatment followed by ICI at time of diagnosis |
R chest wall
Dose: 30
Treatment length: N/a |
Nivolumab (anti-PD1)
Dose: 240mg Treatment length, cycles: N/a |
No |
>10 days from last RT |
10 days after 1st dose |
Grade 3 ICI induced RRD* |
Hydrocortisone 100mg IV, antibiotics and growth factors. Improvement in 1 week. |
N/a |
||
|
Korman 31 |
20’s, Male |
Melanoma of left buttock
Diagnosed 1-year prior ICI treatment |
Left pelvis
Dose: 9 /3 fx
Treatment length: N/a |
Nivolumab (anti-PD1)
Dose, treatment length, cycles: N/a |
1 dose of adjuvant ipilimumab months ago |
10 days from last RT |
3 days after 1st dose b |
ICI induced RRD* |
Paused ICI. Topical corticosteroids. Improvement in 2 weeks. |
N/a |
||
|
Yigit23 |
61, Female |
SCC of unknown primary with metastases to the neck
Initial diagnosis was 1 year and approximately 8 months prior start of ICI |
Left neck level 1-2: 64
Level 3: 60
Level 4-5: 57
Right neck level 2-4: 54
Left parotid: 40
Right neck level 1: 60
Left neck: 40
Total dose: 375 Gy
Treatment length:1.5 year
|
Nivolumab (anti-PD1)
Dose, treatment length, cycles: N/a |
No |
4 months from last RT |
4 weeks after last dose
|
ICI induced RRD* |
ICI not stopped. Topical steroid and oral anti-histamine. Resolved in 2 weeks. |
subacute spongiotic dermatitis
|
||
|
Billena32 |
Female |
Invasive ductal carcinoma of the breast
Initial date of diagnosis: N/a |
Chest wall
Dose: 50/25 fx
Treatment length: N/a |
Nivolumab (anti-PD1)
Dose, treatment length, cycles: N/a |
No |
5 weeks from last RT |
1 week after 1st dose |
ICI induced RRD* |
ICI paused. Topical steroid. |
N/a |
||
|
Vaccaro33 |
55, Female |
Cutaneous SCC of left lower eyelid
Staging N/a
Initially diagnosed 4 years prior starting ICI treatment |
Left lower eyelid
Dose: 66/30 fx
Treatment length: 2 months
|
Cemiplimab (anti-PD1)
Dose: 350mg/3 weeks Treatment length: 42 days Cycles: 3 |
No |
Approx. 4 months from last RT |
4-5 weeks after 1st dose |
ICI induced RRD* |
Topical steroid, emollient cream, nicotinamide, oral anti-histamine. ICI paused. Improvement in 2 weeks |
N/a |
||
|
Wang34 |
52, Male |
SCLC, stage IV
Initial diagnosis 7.5 weeks prior starting ICI treatment |
Lung lesion, mediastinal and supraclavicular nodes
Dose: 50/20 fx
Treatment length: 5 weeks
|
Pembrolizumab (anti-PD1)
Dose: 200mg Cycles: 1 |
Etoposide and cisplatin
|
6 months from last RT |
3 days after 1st dose |
ICI induced RRD* |
Oral steroid |
N/a |
||
|
Deutsch35 |
N/a |
Cutaneous Melanoma (location N/a)
Timing of diagnosis: N/a |
Site: N/a
Dose: 60/30 fx
Treatment length: N/a |
Nivolumab (anti-PD1) Dose, treatment length, cycles: N/a |
No |
>44 months from last RT |
3.9 weeks after 1st dose |
Grade 1 ICI induced RRD* |
none |
N/a |
||
|
Deutsch35 |
N/a |
Cutaneous Melanoma (location N/a)
Timing of diagnosis: N/a) |
Site: N/a
Dose: 20/5 fx
Treatmen length: N/a |
Nivolumab (anti-PD1) dose and treatment length N/a |
Ipilimumab |
>1.5 months from last RT |
3 weeks after 1st dose |
Grade 1 ICI induced RRD* |
Drug d/c due to other irAE’s |
N/a |
||
|
Deutsch35 |
N/a |
Head and neck SCC
Timing of diagnosis: N/a |
Dose: 66/55 fx
Treatment length: N/a |
Nivolumab (anti-PD1) dose and treatment length N/a |
Lirilimumab |
>27 months from last RT |
110 weeks after 1st dose |
Grade 1 ICI induced RRD* |
none |
N/a |
||
|
ICI therapy started while on RT
|
||||||||||||
|
Mesko36
|
70, Female |
Vulvar/ vaginal melanoma
Diagnosed 4 months prior starting treatment |
Vulva
Dose: 63 /35 fx
Treatment length: See ‘Time interval between RT and skin reaction’ column) |
Ipilimumab (anti-CTLA4) Dose: 3mg/kg/3 weeks Length: 6 weeks Cycles completed: 2 |
No |
28 days from start of RT: Current dose 36Gy
40 days from start of RT: Current dose 48.6 Gy |
21 days after 1st dose: Grade 2 cutaneous skin reaction
10 days after 2nd dose: grade 3 cutaneous skin reaction |
Moist desquamation extending beyond radiation fieldC
|
RT and ICI paused for one month. 0.1% topical triamcinolone cream along with a methyl-prednisone dosepak. Improvement in 2 weeks.
|
spongiotic and interface dermatitis with a perivascular inflammatory infiltrate consisting of numerous eosinophils, consistent with a fixed drug eruption. |
||
|
|
RT started while on ICI
|
|||||||||||
|
Eryılmaz37 |
53, Female
Reported as previously healthy
|
Melanoma (right axilla)
Diagnosed 3 months prior starting ICI treatment |
Right axilla
Dose: 30
Treatment length: 10 days |
Ipilimumab (anti-CTLA4) Dose: 3mg/kg/3 weeks Time: 6 weeks Cycles completed: 2 |
IFN-alpha and vemurafenib |
4 days from start of RT |
31 days after 1st dose; 10 days after 2nd dose |
Grade 3 maculopapular rash* |
1 mg/kg oral methylprednisolone. ICI discontinued. Improvement in 3 days. |
Biopsy revealed perivascular eosinophilic mononuclear inflammatory cell infiltration in the dermis with melanin pigment increase in the basal layer. Microscopic features were not compatible with radiodermatitis. |
||
|
Komori25 |
67, Female
Diagnosed at 63 |
Breast cancer, Stage IV
ICI therapy started 18 months after surgery and initial chemotherapy due to liver metastasis |
Mid-back to target lymph nodes in the hepatic portal region
Dose: 30
Treatment length: N/a |
Nivolumab (anti-PD1)
Dose: 2mg/kg/3 weeks Treatment length: 5 months Cycles completed: 7
|
Anastrozole and tegafur-uracil |
5 weeks from last RT |
4 months from first dose |
Lichen planus* |
Difluprednate ointment. ICI d/c/ Complete resolution in 8 weeks. |
Subepidermal lymphocyte infiltrations and a number of necrotic keratinocytes are seen in the epithelium.
|
||
|
Komori 26 |
67, Female
Diagnosed at 63 |
Breast cancer, Stage IV
Re-started RT 8 weeks after first lichen planus reaction |
Cervical vertebrae
Dose: 30
Treatment length: N/a |
Nivolumab (anti-PD1)
Dose: 2mg/kg/3 weeks Treatment length: 3.5 months Cycles completed: 5
|
Anastrozole and tegafur-uracil |
4 weeks from re-starting of RT |
7 months from first dose |
Erosive lichen planus on lower legs |
Topical clobetasol propionate (0.05%) Systemic corticosteroid 20mg/day. Improvement in 4 weeks |
Numerous lymphocyte infiltrations are evident below the epithelium, and a number of necrotic keratinocytes are evident in the epithelium. |
||
|
Katsuo 38 |
76, Female |
Lung adenocarcinoma, stage IV
Started ICI treatment at time of diagnosis |
Whole brain RT
Dose: 28/5 fx
Treatment length: N/a |
Nivolumab (anti-PD1)
Dose: 240mg/2 weeks
Treatment length: 10 weeks Cycles completed: 6 |
No |
4 weeks from last RT |
10 weeks from first dose eruptions spread to all extremities |
Erosive lichen planus on all 4 extremities |
ICI paused. Prednisolone 1mg/kg/day. Rapid clinical improvement |
band-like lymphocytic infiltration beneath an hypertrophic epidermis with compact ortho-hyperkeratosis and hyper-granulosis, as well as focal erosions. Vacuolar changes at the dermo–epidermal junction and apoptotic keratinocytes were also observed |
||
|
Zhao39 |
60, Male |
SCLC, stage IV
Time of diagnosis: N/a |
Lung tumor and mediastinal nodes
Dose: 60/30 fx
Treatment length: N/a |
Nivolumab (anti-PD1)
Dose: 3mg/kg/2 weeks Treatment length: 6 weeks Cycles completed: 3 |
Platinum-based chemotherapy 4 cycles |
Approx. 2 weeks after last RT Current dose 36 Gy/18 fractions
|
4.5 weeks after first dose, (3 days after 3rd dose)
|
Moist desquamation within radiation field* and maculopapular rash (lichenoid reaction^) |
ICI and RT d/c methyl-prednisolone 1mg/kg + infrared ray therapy. Improvement in 10 days
|
N/a |
||
|
Horri40
|
71, Male |
Melanoma of right earlobe
Staging: N/a
Melanoma was resected with local and metastatic recurrence at the earlobe and neck 6 months later |
R earlobe and neck
Dose: 60
Treatment length: N/a |
Pembrolizumab (anti-PD1)
Dose: N/a Treatment length: N/a Cycles completed: 2 |
No |
9 and 11 days after last RT d |
24 days after 2nd dose. |
SJS accentuated in radiation field** |
Pulse methyl-prednisolone and oral prednisone |
Necrosis of the epidermis and junctional detachment
|
||
|
Saw41 |
52, Male |
Sarcomatoid renal cell carcinoma
Started ICI treatment at time of diagnosis
|
T11-L4
Dose and treatment length: N/a |
Pembrolizumab (anti-PD1)
Dose: N/a Treatment length: 56 days Cycles completed: 2 |
No |
2 days after last RT |
77 days after 1st dose, just before 3rd cycle |
SJS accentuated in radiation field* |
ICI d/c Cyclosporine and dexamethasone/levofloxacin eye drops. Resolution in 3 weeks |
Subepidermal vesiculation with epidermal necrolysis and hydropic change associated with a perivascular chronic inflammatory infiltrate of lymphocytes and eosinophils
|
||
|
Hirotsu 42 |
70, Male |
Acral lentiginous melanoma, stage IV
Initial diagnosis approximately 1.5 years prior starting nivolumab. |
Right thigh
Dose: 48
Treatment length: N/a |
Nivolumab (anti-PD1)
Dose: 3mg/kg/2weeks Treatment length: 5.6 months Cycles completed: 13 |
3 cycles ipilimumab, followed by 48Gy RT 8 months later at right inguinal area, followed by 6 cycles of pembrolizumab 7 months after completing RT |
3 weeks after last RT |
29 weeks from first dose |
Bullous pemphigoid * |
D/c ICI. No topical or systemic treatments. Resolution in 1 month. |
Subepidermal blister formation with numerous eosinophils in the blister cavity and a superficial dermal infiltrate consisting primarily of eosinophils. |
||
^ Based on author interpretation of clinical figures, although no biopsy was performed. Maculopapular rash involved 20% of body surface area.
* Cutaneous reaction localized or enhanced at site of RT
** Cutaneous reaction initially localized to site of RT before generalizing
a 5 days - mucosal lesions appeared; 14 days - cutaneous lesions appeared at all radiated sites.
b Erythema started to appear within a few hours after first ICI, officially diagnosed 3 days later due to increasing severity
c Per Mesko et al.36, “the reaction in this patient does not conform well to either radiodermatitis or RDD, it is difficult to discern whether ipilimumab, radiation or simply the combination of the two primarily contributed to the cutaneous toxicities mentioned in this report.”
d 9 days - erythema and erosion of irradiated areas only; 11 days - erythema spread to non-irradiated areas. Per Horri et al.40 RRD developed into SJS
A total of 43% (n=9) cases reported and/or described a skin toxicity of grade 3 or higher, 14% (n=3) reported a skin toxicity of Grade 1, and 57% (n=12) interrupted ICI and/or RT. When ICI therapy was started after completion of RT (n=12), cutaneous reactions occurred in a median of approximately 20 days from the start of ICI therapy and a median of 77 days from the last RT.23,27-35 When RT was started while on ICI therapy (n=9), the cutaneous reactions occurred in a median of 73 days from the start of ICI therapy and a median of 17.5 days from the last RT.25,26,37-42 There was one reported case of a cutaneous reaction occurring when ICI therapy was started while patient was already on RT therapy occurring in 21 and 28 days from start of ICI and RT, respectively.36 No case reports mentioned prior history of skin disease with the exception of Komori et al.26 in which the patient had a previous history of lichen planus secondary to combination therapy as previously reported by Komori et al.25
ICI induced RRD was the most common reported skin toxicity in our literature search (n=10). Median time from first ICI dose to RRD was 15.5 days, median time from last RT to RRD was 77 days. Treatments including interrupting ICI therapy (n=4), systemic steroids (n=3), topical steroids (n=4), and antihistamine (n=1). Most cases (n=4) reported improvement in 2 weeks. Dhanushkodi et al.30 reported using antibiotics and growth factors in addition to IV steroids and noticed improvement in 1 week. An analysis of patient characteristics and type of cancer to determine if there was predisposition to type of cutaneous reaction or severity was performed. SJS occurred exclusively in male patients with no predisposition to age or type of cancer. However, due to the limited number of cases used for this analysis, other significant conclusions could not be confidently interpreted.
Cases of SJS (n=4) involved a PD-1 inhibitor with enhancement at previous sites of radiation. SJS reactions occurred between 7 and 77 days after the first dose of ICI therapy and between 2 days and 4 months of last RT.27,29,40,41 Treatment included discontinuing ICI (n=2), systemic steroids (n=2) and ophthalmic steroids plus antibiotics (n=1). One case did not report if, or if not, ICI therapy was d/c, and one case did not report their treatment plan.29,40 Time to improvement, as reported by Saw et al.41, was 3 weeks.
Lichen planus (n=3) reactions occurred when RT was started while on ICI. The latency period was 10 weeks to 4 months from start of ICI and 4 to 5 weeks from last RT. Treatment included stopping (n=3) ICI therapy, topical and oral steroids. Reactions with moist desquamation (n=2) manifested as localized to site of RT in 1 case (latency 4.5 weeks after first ICI dose and 2 weeks after RT) and extension beyond site of RT in another (latency 3 weeks after first ICI dose, 4 weeks after RT). Treatment included interrupting ICI and RT, topical steroids, systemic steroids, and change to infrared therapy.27,40,41
Lastly, a grade 3 maculopapular rash (n=1), bullous pemphigoid (n=1) appeared exclusively at site of RT during ICI therapy. The maculopapular rash appeared 31 days after first dose of ICI and 4 days from start of RT. Biopsy showed a hypersensitivity pattern with eosinophils rather than features of radiodermatitis.37 ICI therapy was discontinued, systemic steroids were started, and improvement was seen as quickly as 3 days. Bullous pemphigoid (n=1) also remained exclusively at the site of radiation, appearing 29 weeks from first ICI dose and 3 weeks after last RT. Treatment included discontinuing ICI and the bullae healed without any oral or topical treatments.42
Discussion
ICI and RT can be very useful when used together because of their combined ability to control metastatic disease and improve outcomes.7,8 However, cases of RT plus ICI induced skin toxicities have been reported and it is important to be aware of these uncommon, but potentially serious reactions. This review highlights examples of how cutaneous adverse effects manifest during combination RT and ICI therapy, with potential enhancement of toxicity and localization to areas of prior irradiation. Regarding toxicity, previously published large cohorts demonstrated most ICI rashes (10-50%) are associated with lower grade toxicities and approximately only 25% of patients needed to interrupt ICI therapy.15,43 Interestingly, in our review at least 43% (n=9) demonstrated overall higher-grade presentations and 57% (n=12) interrupted ICI and/or RT.
When cutaneous reactions occur primarily at the site of radiation while on ICI therapy, such as ICI-induced RRD and other examples as exemplified by the reports in this review, it is a strong indication that RT and ICI played a synergistic role in the adverse reaction. In this review, 19 cases including diagnoses of ICI induced RRD, whereas cases of SJS, lichen planus, and bullous pemphigoid all initially presented at site of prior RT. 14 cases remained localized and 5 cases generalized to non-irradiated sites. 13 cases reported a response to treatment with topical steroid (n=5), systemic steroid (n=6), combination topical and systemic (n=1), and cyclosporine with ophthalmic drops (n=1). Amongst these 13 cases, 8 interrupted immunotherapy.
One interesting question is whether the sequence of therapy influences the time to onset of cutaneous reactions. In this literature review it was more common for cutaneous reactions to develop sooner when ICI was given after RT (n=12, 57% of cases). Median latency of cutaneous reactions when ICI was given after RT was approximately 20 days after starting ICI. This is in comparison to a median latency of 73 days in patients who received RT after starting ICI (n=8, 38% of cases). Another important consideration is whether these cutaneous reactions were in fact due to combination ICI and RT or rather from either RT or ICI alone, without a synergistic effect. Location, previous reports in literature, and histology should all be considered when determining the cause. RRD, by definition, is a cutaneous reaction at the site of radiation caused by the addition of a medication.22 SJS, lichen planus, and bullous pemphigoid are less commonly reported in the setting of combination RT and ICI therapy. Considering SJS, bullous pemphigoid and lichen planus are far more likely to be attributed to ICI therapy, rather than RT alone,44,45 cases where there was enhancement or localization within irradiated skin suggests a possible synergistic effect.46 Additionally, if the histology of a cutaneous reaction at site of RT does not suggest radiodermatitis, such as in the case by Mesko et al.36, it is reasonable to consider a synergistic etiology.
Although the exact pathophysiology mechanism of the skin toxicities mentioned in this report is unknown, Ruocco's theory of an immunocompromised district (ICD) can help provide an explanation for the above findings. RT damages irradiated skin resulting in chronic changes in the area creating an ICD, or a locus minoris.21 These changes in the skin can result in either a reduced or exaggerated immune response. An exaggerated immunity may lead to development of immune disorders such as lichen planus, bullous pemphigoid, or pemphigus. Ruocco’s theory of ICD also provides insight into the shorter latency of cutaneous reactions in patients who first receive RT followed by ICI. Also considering skin toxicities have been reported with RT in combination with other targeted pharmacological therapies including BRAF, HER2/neu, EGFR, and mTOR, the common factor is the RT.47 Theories proposed by other articles include RT lowering the local threshold of systemic drug reactions and changes to local immune environments after RT, 48,49 which are similair in concept to Ruocco’s theroy of an ICD.
In conclusion, RT can create a locus minoris, predisposing previously irradiated skin to an exaggerated immune reaction when used with ICIs.20,21 In addition to ICI-induced RRD, other skin reactions including SJS site enhancement, lichen planus and bullous pemphigoid have been reported to occur solely at the site of radiation or demonstrate enhancement at the site of radiation. It is important to consider the implications of combining therapies and recognize early or uncommon presentations of inflammatory dermatoses from ICI. However, it is also important to note that although the combination of RT and ICI therapy may exacerbate cutaneous reactions, they do not increase the overall incidence of cutaneous reactions or compromise the efficacy of ICIs.16,23 It can be difficult to conclude if the cutaneous reactions occurred due to the combination of RT and ICI or is solely a cause of the ICI or RT alone. In suspected cases of skin toxicity due to ICI and RT, location, timing, histology, and sequence of therapy, can help provide more insight as well as known associations of certain reactions with either therapy. Using clinicopathological correlations, dermatologists can help identify the etiology, diagnosis, and management of cutaneous adverse effects during RT and ICI and promptly manage such reactions, weighing the risks and benefits of pausing treatment. It is also important to consider that if a reaction to RT and ICI occurs once, there is a probability of re-occurrence with increasing in severity, as seen in the case reported by Komori et al.25,26 Limitations to this paper include only reviewing case reports listed in Scopus and PubMed and the varying amounts of information provided in each case report. Further prospective studies which characterize the morphological type of cutaneous reactions during RT and ICI are needed. This review adds to the growing body of literature synthesizing rare presentations of inflammatory reactions during RT and ICI combination or sequential therapy.
Conflict of Interest
Margaret Kaszycki reports no conflict of interest. Dr. Jonathan Leventhal served on the advisory board for La Roche Posay and Sanofi Regeneron and received clinical trial research funding from Azitra, Inc and OnQuality.
References
- Darvin P, Toor SM, Sasidharan Nair V, et al. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med., Dec 13, 2018; 50(12): 1-11.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med., Nov 29, 2018; 379(22): 2185.
- Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol., Sep 5, 2019; 12(1): 92.
- Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015; 23: 32-38.
- Seidel, J. A., Otsuka, A., & Kabashima, K. (2018). Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Frontiers in oncology. Mar 2018; 28(8): 86.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019; 381(16): 1535-1546. doi:10.1056/NEJMoa1910836
- Stea B, Sirenic T, Hsu CC, et al. The role of radiation therapy in the treatment of metastatic cancer. Clin Exp Metastasis., Aug 2018; 35(5-6): 535-546.
- Liu Y, Dong Y, Kong L, et al. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol., Aug 16, 2018; 11(1): 104.
- Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007; 204(1): 49-55. doi:10.1084/jem.20062056
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006; 203(5): 1259-1271. doi:10.1084/jem.20052494
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. Apr 16, 2015; 520(7547): 373-377.
- Barker CA, Postow MA, Khan SA, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res., Aug 2013; 1(2): 92-98.
- Doyen J, Picard A, Naghavi AO, et al. Clinical Outcomes of Metastatic Melanoma Treated with Checkpoint Inhibitors and Multisite Radiotherapy. JAMA Dermatol. Oct 1, 2017; 153(10): 1056-1059.
- Furue M, Ito T, Wada N, et al. Melanoma and Immune Checkpoint Inhibitors. Curr Oncol Rep. Mar 23, 2018; 20(3): 29
- Tattersall IW, Leventhal JS. Cutaneous Toxicities of Immune Checkpoint Inhibitors: The Role of the Dermatologist. Yale J Biol Med. Mar 27, 2020; 93(1): 123-132.
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. Sep 2017; 56(9): 909-914.
- Bernier J, Bonner J, Vermorken JB, et al. Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol. Jan 2008; 19(1): 142-149.
- Hymes, Sharon R, et al. Radiation dermatitis: clinical presentation, pathophysiology, and treatment. J Am Acad of Dermatol. Jan 2006; vol. 54(1): 28-46.
- Bray, FN, Simmons BJ, Wolfson AH, et al. (2016). Acute and Chronic Cutaneous Reactions to Ionizing Radiation Therapy. Dermatology and therapy, 6(2), 185–206. https://doi.org/10.1007/s13555-016-0120-y
- Piccolo V, Baroni A, Russo T, et al. Ruocco's immunocompromised cutaneous district. Int J Dermatol. Feb 2016; 55(2): 135-141.
- Ruocco V, Ruocco E, Brunetti G. Recall phenomena: another facet of the immunocompromised district. Int J Dermatol. Feb 2013; 52(2): 252-253.
- Azria, Davide, et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev. Nov 2005; 31(7): 555-570.
- Yigit, Ecem, et al. Radiation Recall Dermatitis in Patients Treated with Immune Checkpoint Inhibitors: A Case Report and Literature Review. Cureus Jun 9, 2021; 13(6): e15548
- Yan BY, Wasilewski G, Lacouture ME, et al. Incidence of dermatologic adverse events in patients with cancer treated with concurrent immune checkpoint inhibitors and radiation therapy: A systematic review and meta-analysis. J Am Acad Dermatol. Mar 2021; 84(3): 871-875.
- Komori T, Honda T, Irie H, et al. Lichen Planus in Irradiated Skin During Nivolumab Treatment. Acta Derm Venereol. Mar 10, 2017; 97(3): 391-392.
- Komori T, Honda T, Irie H, et al. Multiple erosive lichen planus preceded by solitary lichen planus after combination therapy with nivolumab and radiation. J Eur Acad Dermatol Venereol. Aug 2017; 31(8): e382-e384.
- Shah KM, Rancour EA, Al-Omari A, et al. Striking enhancement at the site of radiation for nivolumab-induced Stevens-Johnson syndrome. Dermatol Online J. Jun 15, 2018; 24(6): 13030/qt97g3t63v.
- Nakashima K, Saruwatari K, Sato R, et al. Non-small-cell Lung Cancer with Severe Skin Manifestations Related to Radiation Recall Dermatitis after Atezolizumab Treatment. Intern Med. May 1, 2020; 59(9): 1199-1202.
- Rouyer L, Bursztejn AC, Charbit L, et al. Stevens-Johnson syndrome associated with radiation recall dermatitis in a patient treated with nivolumab. Eur J Dermatol. Jun 1, 2018; 28(3): 380-381.
- Dhanushkodi M, Iyer P, Ananthi B, et al. Nivolumab-Induced Radiation Recall Dermatitis (RRD). Indian J Gynecol Oncolog., Feb 20, 2019; 17(21).
- Korman AM, Tyler KH, Kaffenberger BH. Radiation recall dermatitis associated with nivolumab for metastatic malignant melanoma. Int J Dermatol. Apr 4, 2017; 56(4): e75-e77.
- Billena, Cole, et al. “Radiation recall dermatitis after treatment of stage IV breast cancer with nivolumab: a case report.” Immunotherapy vol. 12,2 (2020): 123-130
- Vaccaro, Mario, et al. “Radiation recall during cemiplimab therapy for locally advanced cutaneous squamous cell carcinoma.” Dermatologic therapy vol. 33, 6 (2020): e14417.
- Wang, Yan-Yang, et al. “Concomitant Radiation Recall Dermatitis and Radiation Recall Pneumonitis Induced by Pembrolizumab.” Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer vol. 15,10 (2020): e160-e162.
- Deutsch, Eric, et al. “Can radiation-recall predict long lasting response to immune checkpoint inhibitors?” Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology vol. 154 (2021): 125-127.
- Mesko S, Konecny GE, Tumeh PC, et al. Enhanced skin toxicity with concurrent ipilimumab and radiation in vaginal/vulvar melanoma: a case report and literature review. BJR Case Rep. Jul 2016; 3(1): 20160002.
- Eryılmaz MK, Mutlu H, Salim DK, et al. Ipilimumab may increase the severity of cutenaous toxicity related to radiotherapy. J Oncol Pharm Pract. Jun 2016; 22(3): 533-536.
- Katsuo K, Honda T, Komori T, et al. Erosive Lichen Planus on the Extremities During Combination Therapy with Nivolumab and Radiation: A Second Case Report. Acta Derm Venereol. Mar 31, 2020; 100(6): adv00100.
- Zhao ZM, Liu SC, Xu XJ, et al. Treatment of Skin Reaction Induced by Nivolumab Combined with Radiotherapy in Non-small Cell Lung Cancer: A Case Report. Chin Med Sci J. Sep 20, 2018; 33(3): 183-187.
- Horii M, Kobayashi T, Maeda S, et al. Stevens-Johnson syndrome associated with radiation recall dermatitis in a patient treated with immune checkpoint inhibitor. J Dermatol. Nov 2019; 46(11): e434-e436.
- Saw, Stephanie, et al. “Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients.” European journal of cancer (Oxford, England: 1990) vol. 81 (2017): 237-239.
- Hirotsu K, Chiou AS, Chiang A, et al. Localized bullous pemphigoid in a melanoma patient with dual exposure to PD-1 checkpoint inhibition and radiation therapy. JAAD Case Rep. Aug 30, 2017; 3(5): 404-406.
- Coleman EL, Olamiju B, Leventhal JS. (2020). The life-threatening eruptions of immune checkpoint inhibitor therapy. Clinics in dermatology, 38(1), 94–104.
- Vern-Gross TZ, Kowal-Vern A. Erythema multiforme, Stevens Johnson syndrome, and toxic epidermal necrolysis syndrome in patients undergoing radiation therapy: a literature review. Am J Clin Oncol. Oct 2014; 37(5): 506-513.
- Hadian Y, Chen YC, Eastham DV, et al. Radiation induced lichen planus - an uncommon side effect. Dermatol Online J. Nov 11, 2019; 25(11): 13030/qt6z21r0q7.
- Mul VE, van Geest AJ, Pijls-Johannesma MC, et al. Radiation-induced bullous pemphigoid: a systematic review of an unusual radiation side effect. Radiother Oncol. Jan 2007; 82(1): 5-9.
- Levy A, Hollebecque A, Bourgier C, et al. Targeted therapy-induced radiation recall. Eur J Cancer., May 2013; 49(7): 1662–1668.
- R. Camidge A. Price Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol., Jun 2001; 59(3): 237-245
- Levy A, Chargari C, Cheminant M, et al. Radiation therapy and immunotherapy: implications for a combined cancer treatment. Crit Rev Oncol Hematol. 2012; 85(3): 278-287.
