In vitro Approach to Assess Local Tolerance of Ingredients Dedicated to Specific Topical Care Applications
Alicia Roso1, Mickael Puginier1*, Mathilde Bergal1, Frederic Nunzi2, Alain Alonso3
1Seppic, 50 boulevard National, CS 90020, 92257 La Garenne Colombes Cedex, France
2Groupe IDEA Lab - Site Montesquieu, 5 rue Jacques Monod, 33652 Martillac, France
3EPISKIN, 4 rue Alexander Fleming, 69007 Lyon, France
Abstract
Considering all topical applications, products target very different body areas including mucosa, healthy or impaired skin with many specific characteristics in the epithelium composition, structure and barrier functionality. In vitro reconstructed human tissue models are recognized as being sensitive and reliable in preclinical studies. On top of validated methods for skin irritation, new and predictive experimental protocols can be designed to address specific applications. The objective of this study was therefore to investigate the behavior of ingredients with a well-known tolerance profile for specific applications using 3D human reconstructed models: gingival and vaginal focusing on mucosa tolerance, “immature” epidermis intended to be closer to baby skin, and a fourth epidermis model with a physically impaired barrier function. Ingredients with a key function were applied at usual doses and compared to controls and formulation benchmarks to challenge the predictivity of the models.
The analysis of the results of each in vitro model demonstrated their greater sensitivity compared to the standard reconstructed human epidermis, making it possible to evaluate the tolerance of ingredients and select a well-tolerated dosage according to the local application area. The multiparametric approach designed for the “immature” and “impaired” epithelia models enriched basic irritation information with cellular, morphological and functional effects evaluations. It was therefore possible to identify some infra-clinical reactions and study the ingredient mechanisms.
Context and Objectives
Specific topical applications: a wide range of concerns requiring high tolerance level
The constant quest for innovation, whether through the search for new molecules or the extension to new uses, pushes manufacturers to take tolerance into account in the early stages of development, often going beyond regulatory requirements. Present-day topical applications represent a vast range of formulations, covering multiple product types, people situations, body targets and physiological states, while referring to different laws according to geographical zones. Among the concerns of manufacturers, such as environmental protection, aesthetics and attractiveness, delivery and efficacy, skin tolerance remains an absolute priority, especially when local application targets are far from regular healthy adult skin. Dermo-pharmaceutical or cosmetic producers develop suitable formulations for specific applications onto human external mucous epithelia; such as products for intimate use and oral care, or for other transient disturbed skin states such as after shaving or depilatory products, or sun care products. Cosmetics and dermocosmetics can also be dedicated to sensitive populations, e.g., baby products. In the pharmacy domain, topical administration is increasingly considered to limit some drug side effects and allow for more variable dosage. In dermatology, treatments are often applied on impaired skin barriers, such as for atopic dermatitis or psoriasis1- 3 and wound healing. It is also temporarily the case for products applied after microdermabrasion4 or after tattoos5 and concerns medical devices as well. Even for only beauty-related purposes, the prevalence of people with sensitive or reactive skin, up to approximately 50% of adults according to region6, 7, should be considered. Finally, the current worldwide megatrend of beauty customization and Do-It-Yourself products (DIY) raises an additional challenge for cosmetic manufacturers due to less control over ingredient dosage and final homemade mixtures made directly by the consumers. Unintentional application of the product to other sites of the body during use should also be taken into consideration. In this complex context, it is more and more important to guarantee the tolerance of ingredients within a wide range of possible applications.
From the perspective of the ingredient supplier, assessing the tolerance at early stages of the development of a new ingredient is therefore essential, especially when new structures are designed. The challenge is to find relevant and predictive in vitro models to help in diverse situations.
Objectives of the study
The goal of this study is to go further than regulatory safety testing and investigate the local tolerance on specific targets of key versatile ingredients with a well-known toxicological profile. Ingredients have been tested at typical use levels, compared to controls and benchmark formulas. Different reconstructed human epithelia models have been selected according to the site of application and the skin condition. In a first step, the mucosal tolerance of ingredients has been investigated on existing gingival and vaginal reconstructed mucosal models, using a robust cell viability parameter, based on the existing history of use on these models. In a second step, the ingredients were challenged on two experimental models designed to address “immature” and physically impaired epidermis, hoping to show higher sensitivity compared to standard human reconstructed epidermis. On these last two models, a multiparametric approach was applied, to take into account the mechanism of irritation as a whole and to refine the analysis of the mode of action of the ingredients.
The resulting data are aimed at helping final manufacturers to select well-tolerated ingredients according to the final use and to choose a suitable dosage from the beginning of the development of their formula. This recommended dosage should be in line with the safety evaluation of the ingredient for the specific target which will not be addressed in this work.
A Step Beyond Classical Reconstructed Human Epidermis: The Challenge of Advanced 3D Epithelium Models
Initially driven by regulatory and ethical concerns, the in vitro Reconstructed Human Epithelium model (standard RHE) is recognized as a reliable preclinical tool for assessing the tolerance of cosmetic or personal care products and ingredients8. One of the main advantages of this model is the presence of a barrier layer that allows the topical application at the same doses as in vivo, including poorly water-soluble compounds and formulations, which corresponds to a large number of ingredients used in skin care and dermatological treatments. In addition to useful validated methods for skin irritation, there is a need for the ingredient suppliers to build an in vitro testing set in order to provide more targeted tolerance data when the conditions of use are far from that of normal adult skin. Because tolerance is a complex biological mechanism, consideration of the structure and state of the epithelium (e.g., age, mucosa characteristics or skin barrier integrity) and choosing end-points tailored to specific biological parameters are essential to support the analysis.
Epithelia are perceived as barriers that protect our body from the outside environment and exposure to irritants. Epithelium is a complex structure, composed of different cell types, cell to cell junctions and three-dimensional arrangements to carry out different physiological functions. Various anatomical structures of the epithelia can influence barrier permeability9. Within the stratum corneum, recognized as playing a predominant role, variations such as thickness and number of cell layers can influence the physical barrier effect. The composition, organization and packing of intercellular lipids are other important parameters forming the biochemical barrier that show regional differences, as well as the density of hair follicles and sebaceous glands. For instance, on normal skin of 301 individuals, the number of stratum corneum cell layers was found to vary from a minimum of around 6 in genital epithelium compared to 9 on the face and around 12 or 13 on the scalp and the trunk (mean ± 2 SD), to a maximum of around 47 on the palms and soles10. In full-term babies, the epidermis structure is competent and functional, but noticeable anatomical differences from adults were also reported up to two years after birth, confirming the relative immaturity of the epidermal barrier: 30% thinner stratum corneum with higher surface micro-relief, thicker (in height) corneocytes, but fewer layers, lower cohesion within the structure, and uneven distribution of corneodesmosomes associated with poorly controlled cell turnover. Resulting disturbed water-handling properties and lower lipid content until prepuberty were also reported11-14.
The condition of the epidermis can dramatically influence barrier functionality. Loss of integrity of the stratum corneum, its disruption or partial removal provoked by a mechanical effect or consecutive to skin disorders such as atopic dermatitis or eczema, was seen to increase transepidermal water loss from 5 to 10 times depending on sources15. In a normal stratum corneum, lipids surrounding the corneocytes are arranged in a three-dimensional lamellar bilayer structure, predominantly parallel to the skin surface, often referred to as a Landmann unit. There is wide agreement on the importance of this organization of lipids for a healthy, functional skin barrier. Evidence of a change in the balance of the distribution of intra-lamellar crystalline hexagonal and orthorhombic lipids was observed in lamellar ichthyosis and atopic dermatitis skin diseases, associated with an impaired skin barrier16. Barrier disruption may also stimulate signaling cascades leading to inflammation. Considering the great variety of impairment causes, it has been suggested in previous pharmaceutical studies that more reproducible and simple impairment simulation can be achieved by an artificial mechanical abrasion protocol15.
As for the epidermis, external mucosa mainly targeted in cosmetic and dermo-pharmaceutical applications, i.e., oral and vaginal, comprise a covering epithelium that acts as a barrier against exogenous substances and pathogens. Compared to normal skin, even if oral and vaginal mucosa are also stratified squamous epithelia that looks structurally like the epidermis, fundamental differences were reported in line with more or less barrier function efficacy. The stratum corneum of the vaginal mucosa is predominantly non-keratinized. Vaginal stratum corneum indeed comprises loosely connected glycogen-filled cells17. Intercellular stacked lipid lamellae were identified but without reaching to form an impermeable intercellular lipid envelope as they do in the epidermis, thus leading to a lower barrier function17. The influence of lipid composition was not fully investigated. The structure of the external part of oral mucosa varies depending on the location as well as the permeability barrier function. In the regions of the hard palate and gingiva, the epithelium is keratinized like the epidermis, in which most of the cells are keratinocytes. Even if the stratum corneum of gingival epithelium provides a permeability barrier, differences in intercellular lipid composition and conformation induce a less ordered and packed structure, and thus a less efficient barrier than the stratum corneum of the epidermis18. In the buccal region, lining mucosa inside the lips and cheeks, on the floor of the mouth and the underside of the tongue is a nonkeratinized epithelium, characterized by a less effective barrier function. Buccal mucosa has been reported to have structural similarities with the vaginal mucosa18, 19 with comparable barrier properties demonstrated in various ex vivo penetration studies conducted between 1998 and 2001 by P. Van der Bijl and A. D. Van Eyk’s research team. This suggested that human vaginal mucosa can be used as a model for buccal mucosa in studies of permeability to various chemical compounds20-23.
However, considering the great diversity of epithelia, it makes sense to anticipate and evaluate the local tolerance of ingredients on biological systems closer to the final application sites.
Looking at the subject from the perspective of what are the relevant parameters to be assessed, skin irritation involves a cascade of physiological responses at molecular, cellular and tissue level. In vivo, the end phenomenon, i.e., tissue damage, mainly erythema and oedema, are recorded as signs of irritation. As the vascularization effect cannot be reached in vitro, cellular viability was considered as a readout of cell and tissue damage and was demonstrated to be the most robust endpoint, correlated with in vivo irritation in standard human reconstructed epidermis (OECD guideline N° 43924) and based on numerous studies involving both cosmetic formulations and soluble to insoluble ingredients e.g.25, 26. Beyond universal cellular viability assessment, in vitro models offer the possibility of measuring phenomena occurring earlier in the cascade of events thus giving access to a better understanding of the mechanism of each topically-applied substance. Before affecting cell viability, the substance can interact through interconnected pathways involving disturbance of the tissue integrity and inflammation, resulting in a decreased barrier function followed by substance penetration with potential cytotoxic effect. Barrier function disturbance and substance penetration through the epithelium were described to be successfully followed by decreasing Transepithelial Electrical Resistance (TEER). Release of lactate dehydrogenase-LDH enzyme was reported to be a relevant marker of cellular membrane damage, and release of cytokine Interleukin-1 was highlighted as one of the essential inflammation signals, at the root of inflammatory reactions, that can occur at non cytotoxic doses27, 28. Finally, histology observations provided evidence of tissue integrity. This cascade of physiological responses pointed to the interest of a multiparametric assessment.
Some previous publications have discussed evaluations of local tolerance of specific topical formulations using in vitro models. For example, trials on baby formulations applied on proprietary human reconstructed immature epidermis in comparison with the proprietary adult model revealed a significant difference in cellular viability with decreased values for the immature model29, 30. The interest of immature, not fully differentiated, reconstructed epidermis in assessing the effect of hard water alone or with cleansers was also demonstrated using a multiparametric analysis31. In oral care, evaluation of teeth whitening formulas using human reconstructed gingival epithelium and oral epithelium showed a cytotoxic effect in both models according to hydrogen peroxide content, with a higher sensitivity of oral mucosa to irritants, in line with the absence of a cornified layer32. The irritation potential of intimate care products was also investigated on human vaginal-ectocervical models with the ability to distinguish the irritancy levels of various commercially available formulations measuring the exposure time required to reduce cellular viability below 50%33. A good correlation between the in vitro human vaginal epithelium model and in vivo results was reported on five intimate hygiene cosmetic products34. However, there is little data dealing with experience on ingredients.
Selected in vitro Models
In order to provide more consistency in the analysis, the quantity of ingredient applied at the epithelial surface was chosen to be identical in the four protocols. The concentration of ingredient per unit area was selected within the range recommended in the OECD 439 testing guideline24 and close to the one generally used in human patch tests, i.e. 30 µL/ cm2. With the same concern for consistency, cellular viability was assessed on all the in vitro models, using the same protocol and controls for calibration.
For the two existing mucosal models, the protocols were inspired by epithelium 3D model suppliers, as well as the analysis of results, which was based on an adopted scale of single cell viability. On the contrary, with the two new experimental models developed in partnership with expert CRO (Contract Research Organization) companies, several tailored end-points have been explored.
Gingival tolerance
- Description of the model
The biological system is based on cultivated human normal gingival cells and is histologically similar to the outer cell layers of the human gum (SkinEthic™ HGE model from EpiSkin company shown in figure 1).
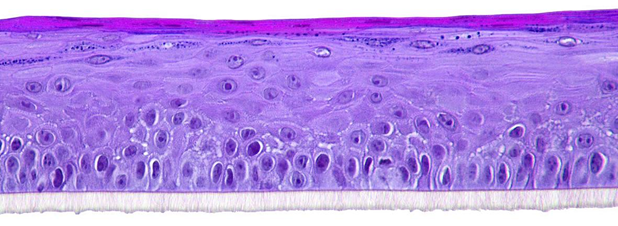
Figure 1: Reconstructed gingival epithelium; Skin Ethic™ HGE;
Histological section, H&E staining; Photo magnification x 20 © Episkin company.
- Protocol
Duplicate protocol was followed according to IDEA Lab standard protocol. Cell viability was assessed after application of 30µl of the test item at the surface of the mucosa for four contact times: 10 minutes, 1 hour, 3 hours and 24 hours by measuring the activity of the mitochondrial succinate dehydrogenase which converts MTT into blue formazan crystal. After 30 minutes incubation with the MTT, (3-(4,5-dimethylthiazol-2-yl) -2,5- diphenyltetrazolium bromide), the color of each culture was recorded. A blue color indicated that the cells were alive, a white color that they were dead. Quantified results were obtained by spectrophotometric measurement after blue formazan crystal dissolution; the measured absorbance at 540nm is proportional to the number of living cells. The test was validated by a negative control (Phosphate Buffered Saline = PBS) that must be classified as non irritant and a positive control SDS (Sodium Dodecyl Sulfate) classified as slightly irritant to irritant at 0.5% and irritant at 1%. The results were expressed as a percentage of viability compared to the negative control: Viability % = Test item Absorbance / Negative control Absorbance. A lower viability than 50% corresponds to a predominant white epithelium whereas a higher viability than 50% corresponds to a predominantly blue epithelium. The irritant potentials were established according to the indicative protocol developed by Skinethic™ (1992-1996) in consideration with the exposure time that reduces the tissue viability to 50% (Scale described in Table 1). This historic time-to-toxicity principle is more than ever relevant to increase predictive capacity35
Table 1: Tolerance analysis
|
Cellular Viability (% versus negative control) |
Conclusion |
|||
|
10 minutes |
1 hour |
3 hours |
24 hours |
|
|
> 50% |
> 50% |
> 50% |
> 50% |
Non irritant |
|
> 50% |
> 50% |
> 50% |
< 50% |
Very Slightly irritant |
|
> 50% |
> 50% |
< 50% |
< 50% |
Slightly irritant |
|
> 50% |
< 50% |
< 50% |
< 50% |
Irritant |
|
< 50% |
< 50% |
< 50% |
< 50% |
Very irritant |
Vaginal tolerance
- Description of the model
The biological system is based on cultivated A431 cells derived from a vulva epidermoid carcinoma. The model is histologically similar to vaginal mucosa (SkinEthic® HVE model from EpiSkin company shown in figure 2).
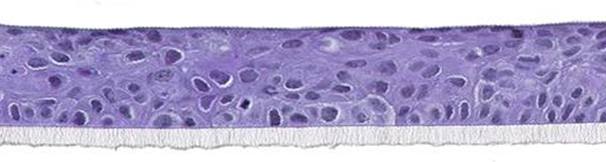
Figure 2: Reconstructed vaginal epithelium; SkinEthic® HVE
Histological section, H&E staining; Photo magnification x 20 © Episkin company.
- Protocol
A duplicate test was performed according to IDEA Lab standard protocol. Cell viability was assessed after application of 30 µl of the test item at the surface of the epithelium for four contact times: 10 minutes, 1 hour, 3 hours and 24 hours. The measurement principle and the results were analyzed and expressed in the same manner as for the reconstructed gingival epithelium described in paragraph 2.1.
A duplicate test was performed according to IDEA Lab standard protocol. Cell viability was assessed after application of 30 µl of the test item at the surface of the epithelium for four contact times: 10 minutes, 1 hour, 3 hours and 24 hours. The measurement principle and the results were analyzed and expressed in the same manner as for the reconstructed gingival epithelium described in paragraph 2.1.
Skin tolerance according to epidermis states
Normal mature Skin tolerance
- Description of the model
A standard reconstructed human epidermis model from normal human keratinocytes (SkinEthic® RHE) was used as a reference in the design of the “immature” and “impaired” experimental models. RHE is formed after 17 days of culture on an inert polycarbonate filter at the air-liquid interface, in a chemically defined medium. The model was demonstrated to be histologically similar to in vivo human epidermis with the presence of major epidermal lipid classes and characteristic bi-layered lipid lamellae in the intercellular space of the stratum corneum. The reconstructed human epidermis was also proven to express the major differentiation markers in the different cell layers such as filaggrin, keratin 5 and 10, and loricrin.
Controls were tested on RHE models in comparison with the “immature” and “impaired” experimental models to challenge their sensitivity and their ability to better discriminate the irritation potential of ingredients.
- Protocol
30 µL of the control was directly applied for a maximized 24-h treatment and then washed off. Cellular viability was assessed using the same MTT testing protocol as for the mucosal models (see 2.1 and 2.2). As a sole maximized time of contact was involved, the product was classified as non-irritant if the viability was above 50% (corresponding to a predominant blue epithelium) and considered irritant if the viability was lower than 50% (corresponding to a predominant white epithelium as illustrated in figure 3).
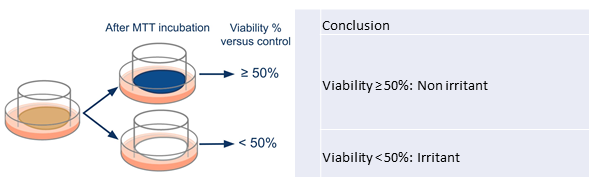
Figure 3: Cytotoxicity analysis for reconstructed human epidermis based on MTT test
Young/ Baby skin tolerance
- Description of the model
Histologically close to the classic reconstructed epidermis, the biological system is characterized by a shorter cultured state (SkinEthic® RHE model at 10 days, instead of 17 days for a standard model) in order to obtain a less differentiated epidermis. A 10-day culture leads to lower barrier functions, resulting in epithelium expected to be more susceptible to irritation phenomena.
- Protocol
The protocol was developed in partnership with IDEA Lab. Cell viability was assessed after application of 30 µl of the test item at the surface of the epidermis (duplicate test performed; no statistical analysis performed) and after a maximized 16 hours of contact (this time was chosen to keep realistic conditions versus the final use and to be coherent with the tested benchmark results). MTT testing protocol and results analysis were identical to standard reconstructed epidermis (2.3 - figure 3).
In order to improve the performance of the test, the inflammatory response through IL-1α release was quantified in the culture media by immunoenzymatic assay (Quantikine® Human IL-1α Immunoassay kit, results expressed in pg/ml) and a supplementary epidermis was treated for histological analysis (paraffin included slides, hematoxylin/eosin: H&E staining). Damage was scored according to its occurrence in each of three epidermal layers, from 0 = absent, to 1 = rare, to 2 = moderate, to 3 = marked compared to the negative and positive controls. Picnosis and perinuclear vacuolization were scored both in the basal layer and in the granular layer, and ballooning was scored in the corneal layer. A global score corresponding to the sum of the effects on the three layers was calculated (from 0 to 15 for marked damage; see examples of corresponding epithelium states in figure 4). A tentative final conclusion was established relative to the controls according to each parameter: cytotoxicity, IL-1α release, and histological scoring. It was considered a good tolerance when viability was superior to 50% and the other parameters were comparable to the negative control. The tolerance was considered to be acceptable (also considering the benchmark results) when viability was superior to 50% with concomitant slight to moderate effects on inflammation and/or histology, compared to the negative control. Finally, the tolerance was considered poor when viability was below 50%, indicating an irritant effect, or when inflammation and tissue damage were considered at least as marked or extreme compared with negative control.
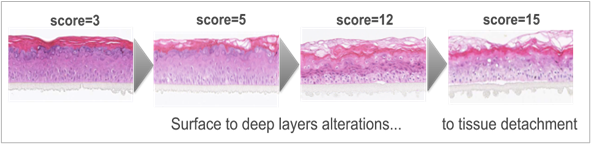
Figure 4: Illustration of increasing tissue alterations according to histology scoring (sum of damage in each layer; Histological section, H&E staining).
“Impaired skin” tolerance:
- Description of the biological system
Reconstructed Human Epidermis (SkinEthic® RHE) on which the stratum corneum surface was mechanically abraded to impair the barrier function (figure 5) and increase sensitivity to irritation (experiments conducted in partnership with VitroScreen, in vitro research laboratory, Milan Italy). The controlled abrasion procedure, performed using a surgical device, was optimized to avoid inducing an inflammatory process in the viable epidermis and significant toxicity. Trans-Epithelial-Electrical-Resistance was checked before and after abrasion; a reduction of TEER value around 50% is induced by the mechanical abrasion, stable 24 hours after impairment36.
- Protocol
The study was conducted on triplicate RhE. After impairment on the epidermal surface, 30 µL of the test item was directly applied for a maximized 24-h treatment and then washed off. Four parameters were then evaluated according to Multiple Endpoints Analysis (MEA37) Cellular viability was firstly assessed by the MTT test in order to quantify the toxicity of the ingredients. Overall skin barrier functionality was followed by TEER measurement versus impaired baseline (in ohm*cm2) by TEER that measures the movement of ions across the paracellular pathway regulated by polarized plasma membrane surfaces and by cell-to-cell tight junctions that together modulate the movement of solutes and ions across the epithelium: it reflects the global integrity of the epidermis barrier, linked both to the structure and to epithelium thickness. A complementary biotin permeability assay was conducted for a deeper understanding of the interaction between the product and the living epithelial tissue after 24 h treatment. Paraffin included slides were stained by secondary antibody Texas Red Streptavidin Conjugate staining + DAPI for nuclei/ Fluorescent microscopy analysis with Leica DM200 FLUO. Biotin is a vitamin which is used as a tracker to evaluate the integrity of living epidermis in an inside-out permeation model focusing the tight junctions structure at granular layer. Tissues were incubated with a biotin solution introduced in the basal chamber (1 h at 37°C) and its penetration into the tissues was monitored in comparison to the negative control (saline buffer). The results were scored by a pass/ fail approach as YES/NO biotin permeation in the RhE. Lastly, Histo-morphological analysis was performed (paraffin included slides and H&E staining/ Light microscopy analysis with Leica DM200 FLUO) and effects were scored from 0 to 4 according to depth of alterations versus the negative control (0 = no significant modification; 1 = slight modification limited to stratum corneum = SC; 2 = moderate modification in SC and granular layer; 3 = strong modification at basal layer such as necrotic cells, intercellular holes, edema; 4 = severe modification leading to a loose structure).
The test was calibrated by a negative control (Phosphate Buffered Saline = PBS) that must be classified as non-irritant and a positive control SDS (Sodium Dodecyl Sulfate) classified as irritant at 0.25% and non-irritant at 0.15% but with a highly significant effect on the other parameters.
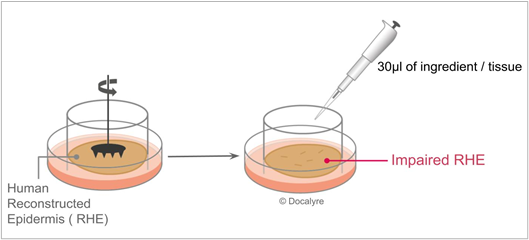
Figure 5: Impaired RHE biological system and exposure protocol.
A tentative prediction was set up relative to the negative control and according to each parameter: cytotoxicity, TEER, histological scoring and Biotin permeation. The tolerance was considered good when viability was superior to 50% and the other parameters were not significantly affected compared with the negative control. The tolerance was considered to be acceptable (also considering the benchmark results) when viability was superior to 50% and other parameters did not provide marked effects compared with the negative control. Tolerance was deemed poor when viability was below 50% indicating an irritant effect or when consistent and extreme effects on TEER, histology and biotin migration reflected a complete loss of the epithelium barrier function.
Selection of Ingredients
One way of ensuring a good tolerance of formulations dedicated to specific applications is of course to select the ingredients and their dosage carefully, and also to control their numbers in order to design minimalist compositions that minimize the risk of interactions between ingredients. With this in mind, ingredients should be versatile and very powerful to guarantee the stability and efficacy of the formula while providing suitable aesthetics. Appearance and skin feel, especially spreadability, were demonstrated to be an integral part of efficacy and long-term adherence of cosmetics38,39 and dermatological products40. A selection of pillar ingredients with different chemical structures and multiple functionality were chosen in accordance with this perspective (shown in table 2): thickening-stabilizing-texturing rheology modifiers with different sensory profiles41 to create aqueous gels, cream gels and emulsions; mild glucolipid-based surfactants with emulsifying42,43 and solubilizing properties, a sugar-based moisturizing active ingredient44 and two plant-based active ingredients (soothing and repairing). All these ingredients are well known to provide good skin tolerance. They were tested at a realistic efficient-cost dose, in simple dilutions, on all the models or only on the most appropriate, according to their functionality, skin needs and formulation habits in the specific application under consideration. For example, a conservative approach was adopted for active ingredients in baby products, with a focus on the essential need of moisturizer whereas skin repairing and soothing active ingredients were tested more broadly. For oral care, the interest of a plant-based solubilizer was suggested by the frequent use of clear formulations like mouthwash or gel treatments. Likewise, since in oral care and intimate care many products are clear or translucent, Polymers R.M. 1, 2 and 4 allowing this suitable appearance were selected. On the contrary, R.M. 3, giving a white appearance even in aqueous gel, was added in view of baby care and impaired skin applications in which emulsions and white appearance are widely represented.
In addition to common negative and positive controls, one in-house control substance with a known dose-effect response from non-irritant to moderately irritant for skin was evaluated in order to challenge the sensitivity of the models (Control-IC; experimental substance not available on the market).
Some formulation benchmarks with high tolerance targets, described in table 3, were also included in the evaluation as comparators and in order to calibrate the analysis of the results in the corresponding specific application. These formulas were tested according to their real conditions of use, i.e. pure for leave-on applications and at 10% dilution in case of a rinse-off application.
Table 2: Description of ingredients and targeted specific applications
|
Category |
Function/ code |
Structure/ INCI |
Specific applications |
Market targets |
||||
|
Oral care |
Intimate use |
Baby product |
Impaired skin |
Cosmetic |
Dermo- Pharmacy |
|||
|
Active ingredient |
Moisturizer |
Xylityl Glucoside & Anhydroxylitol & Xylitol |
• |
• |
• |
• |
â |
|
|
Skin repair |
Asiaticoside acid & Madecassic Acid & Asiatic Acid |
• | • |
|
• |
â |
â |
|
|
Soothing |
Madecassoside |
|
• |
|
• |
â |
|
|
|
Surfactant |
O/W Emulsifier 1 |
C 20-22 Alcohols & C 20 Glucoside |
• | • | • | • |
â |
|
|
O/W Emulsifier 2 |
C 16-18 Alcohol & C16-18 Glucoside |
• | • | • | • |
â |
â |
|
|
Non foaming solubilizer |
Heptyl Glucoside |
• |
|
|
|
â |
|
|
|
Rheology modifier |
R.M. 1 |
Hydroxyethyl Acrylate & Sodium Acryloyldimethyl Taurate Copolymer |
• | • | • | • |
â |
â |
|
R.M. 2 |
Polyacrylate Crosspolymer-6 |
• | • | • | • |
â |
â |
|
|
R.M. 3 |
Hydroxyethyl Acrylate & Sodium Acryloyldimethyl Taurate Copolymer & Squalane & Polysorbate 60 |
|
|
• | • |
â |
|
|
|
R.M. 4 |
Acacia Gum & Xanthan gum |
• | • |
|
|
â |
|
|
|
Controls |
Negative control |
Phosphate-Buffered Saline (PBS) |
Used to validate the results in all the models |
Not applicable |
||||
|
Positive control |
Sodium Dodecyl Sulfate (SDS) |
|||||||
|
In-house positive Control-IC |
Anionic Experimental Surfactant structure C12 |
|||||||
Table 3: Characteristics of benchmark formulas
|
Formula |
Type |
Composition Main components: surfactant / oil / rheology modifier |
Specific application |
|
Benchmark 1 |
Solution |
Alcohol, sodium benzoate, sodium monofluorophosphate, benzoic acid |
Oral care |
|
Benchmark 2 |
Cleansing solution |
Lactic acid, magnesium laureth sulfate, disodium laureth sulfosuccinate, cocamidopropyl betaine, sodium laureth sulfate |
Intimate use |
|
Benchmark 3 |
Cleansing solution |
PEG-80 Hydrogenated Glyceryl Palmate, Cocamidopropyl Betaine, Magnesium Laureth Sulfate, Polysorbate-20, Sodium Cocoyl Aminoacids, Disodium Laureth Sulfosuccinate |
|
|
Benchmark 4 |
O/W emulsion |
Stearic Acid/ Glyceryl Stearate/ Paraffinum Liquidum/ Carbomer |
Baby care |
|
Benchmark 5 |
O/W emulsion |
Glyceryl Stearate/ Ceteareth-20/ Paraffinum Liquidum/ Carbomer |
|
|
Benchmark 6 |
Oil |
Mineral Oil |
|
|
Benchmark 7 |
Gelled Oil |
Mineral Oil/ Hexyl Laurate/ Hydrogenated Styrene/ Isoprene Copolymer/ Cyclopentasiloxane |
|
|
Benchmark 8 |
O/W emulsion |
Glycerol/ Paraffin Oil/ Vaseline/ Glyceryl Monostearate/ Stearic acid/ Macrogol 600 |
Damaged skin |
Ingredients and Benchmark Tolerance on 3D Models
Results and discussion on reconstructed gingival epithelium
As shown in table 4, preliminary trials on positive SDS control demonstrated that a dose of 1% was necessary to detect an obvious indisputable irritant effect on reconstructed gingival epithelium. These findings are consistent with previous publications using 0.5% of SDS and highlighting the lack of effect on cell viability32, 45. The in-house positive control-IC, tested at a usual dose for such a structure, induced a strong decrease in cell viability at 24 h of contact despite being perfectly well tolerated in the human 48-h patch test at the same dosage on skin. This result shows the interest of this organotypic model to refine the tolerance evaluation of ingredients in order to select those to use preferentially in formulas in contact with intimate areas.
The benchmark formula and the ingredients tested at realistic efficacious use levels were well tolerated with a cell viability maintained above 50% after 24 h of contact, except for the skin repair active ingredient that provided two inconsistent results, close to the threshold. The first trial provided a cell viability at 24 h within the limit of data interpretation (50% ± 5%) while the second showed a significant effect, leading this active ingredient to be considered as ‘Non-irritant to Very slightly irritant’ according to the scale adopted. These results showing big variations could be linked to the occlusivity provided by the vehicle, paraffin oil, chosen for solubilization purposes, and need further investigation.
A simple cell viability analysis was chosen in the first instance, but the results observed with the skin repair active ingredient highlighted the interest in monitoring complementary parameters to consolidate the conclusion. Histology observations, for instance, were suggested by other authors as a relevant parameter to evaluate the tolerance of oral care formulations, whereas inflammatory marker IL-1Α release was found to be correlated with cell viability reduction32. IL-1Α release instead was found in another study to be a good indicator of acute irritation of the positive control SDS at 0.5%45, offering an interesting prospect for improving the predictivity of the model, at least for the evaluation of surfactant structures.
Table 4:Results of Reconstructed gingival epithelium model
|
Products |
Dose (wt%) |
Cellular viability (%) |
Conclusion |
||||
|
10 minutes |
1 hour |
3 hours |
24 hours |
||||
|
Controls |
Negative control PBS |
|
100 |
100 |
100 |
100 |
Non Irritant |
|
Positive control SDS |
0.5a |
Not tested |
92 |
88 |
Not tested |
Non irritant or very slightly irritant |
|
|
1a |
92 (mean; n=5) |
61 (mean; n=5) |
37 (mean ; n=5) |
8 (mean ; n=5) |
Slightly Irritant
Model validation criterion=Very Slightly Irritant to Slightly Irritant |
||
|
Positive control-IC |
1.5a |
85 |
85 |
83 |
7 |
Very Slightly Irritant |
|
|
Benchmark |
Benchmark 1 |
Dilution 1/10a |
99 |
100 |
100 |
76 |
Non Irritant |
|
Ingredients |
Moisturizer |
3a |
80 |
85 |
79 |
75 |
Non Irritant |
|
Skin repair active |
1c |
100 |
95 |
95 |
51-36 |
Non Irritant to very slightly irritant |
|
|
Emulsifier 1 |
2a |
100 |
92 |
92 |
67 |
Non Irritant |
|
|
Emulsifier 2 |
5b |
100 |
95 |
93 |
87 |
Non Irritant |
|
|
Solubilizer |
5a |
86 |
100 |
100 |
68 |
Non Irritant |
|
|
R.M. 1 |
3a |
100 |
100 |
100 |
84 |
Non Irritant |
|
|
R.M. 2 |
2.5a |
100 |
100 |
100 |
95 |
Non Irritant |
|
|
R.M. 4 |
2a |
100 |
98 |
88 |
74 |
Non Irritant |
|
aIn demineralized water at pH≅6.3-7, bIn demineralized water + 5% Glycerin, cIn paraffin oil
Results on reconstructed vaginal epithelium
Irritancy of SDS with dose-effect on the reconstructed vaginal epithelium was perfectly identified by cell viability kinetic measurements (table 5). A dose-effect response was also observed with the in-house positive control-IC showing a good tolerance at 0.3% but a strong decrease in cell viability after 24 h of contact for the 1.5% dose. At 3% a decreasing viability trend can also be observed with control-IC at 3 h, indicating that a slight effect can be detected thanks to the kinetics measurements. Intimate benchmark 2 showed significant reduction of cell viability after 3 h of contact, and benchmark 3 at 24 h of contact. These results on two well-accepted dermatological products show the high sensitivity of the vaginal epithelium and its ability to maximize the potential effect of ingredients. All the selected ingredients, tested at realistic dosage, showed a good tolerance according to the adopted scale. Among them, rheology modifier 4 induced a significant reduction of cell viability at 24 h next to the threshold limit. However, as this effect was not obviously observed on the gingival epithelium, it could be associated with the specific characteristics of the vaginal epithelium. As highlighted in the assay on gingival epithelium, this emphasized the usefulness of other responsive parameters to support the ingredient mechanism and the global conclusion. As an inspiring example, a study on another human vaginal reconstructed model substantiated TEER measurement, histology and cytokine release to understand the interactions of a well-known spermicidal agent with vaginal tissue46. Results on intimate cleansing formulations also evidenced the interest of complementary TEER measurement, whereas IL-1α inflammatory marker was mostly found correlated with cell viability34. Another area of study could be to challenge the ingredients on a lactobacillus species colonized vaginal mucosa in order to be closer to realistic conditions as suggested for the study of pre- and pro-biotic ingredients in a previous publication47.
Table 5:Results of Reconstructed vaginal epithelia model
|
Products |
Dose (wt%) |
Cellular viability (%) |
Conclusion |
||||
|
10 minutes |
1 hour |
3 hours |
24 hours |
||||
|
Controls |
Negative control PBS |
|
100 |
100 |
100 |
100 |
Non Irritant |
|
Positive control SDS |
0.5a |
81 (mean ; n=8) |
42 (mean ; n=8) |
11 (mean ; n=8) |
4 (mean ; n=8) |
Irritant |
|
|
1a |
82 (mean; n=4) |
13 (mean; n=4) |
Not tested |
Not tested |
Irritant Model validation criterion=Very Slightly Irritant to Slightly Irritant |
||
|
Positive control-IC |
0.3a |
100 |
100 |
100 |
100 |
Non irritant |
|
|
1.5a |
100 |
100 |
100 |
5 |
Very Slightly Irritant |
||
|
3a |
100 |
100 |
64 |
1 |
Very Slightly Irritant |
||
|
Benchmarks |
Benchmark 2 |
Dilution 1/10a |
100 |
100 |
42 |
4 |
Very Slightly to Slightly Irritant |
|
Benchmark 3 |
Dilution 1/10a |
100 |
100 |
85 |
2 |
Very Slightly Irritant |
|
|
Ingredients |
Moisturizer |
3a |
100 |
100 |
100 |
85 |
Non Irritant |
|
Skin repair active |
1b |
97 |
99 |
95 |
85 |
Non Irritant |
|
|
Soothing active |
0.5a |
89 |
89 |
82 |
76 |
Non Irritant |
|
|
Emulsifier 1 |
2* |
89 |
100 |
100 |
88 |
Non Irritant |
|
|
Emulsifier 2 |
5c |
92 |
100 |
100 |
92 |
Non Irritant |
|
|
R.M. 1 |
3a |
100 |
100 |
100 |
100 |
Non Irritant |
|
|
R.M. 2 |
1a |
100 |
100 |
100 |
100 |
Non Irritant |
|
|
2a |
100 |
96 |
95 |
96 |
Non Irritant |
||
|
R.M. 4 |
1a |
100 |
100 |
100 |
51 |
Non Irritant - Limit |
|
aIn demineralized water at pH≅6.3-7, bIn paraffin oil, c5% Paraffin oil + water
Results on experimental reconstructed “immature” epidermis
- Validation of model sensitivity versus standard Reconstructed Epidermis (RHE)
The cytotoxicity comparison between the in-house positive control (Control-IC) and a dose-response effect between the “Immature” and the standard RHE model evidenced the higher sensitivity of the “immature” models (figure 6). Control-IC was indeed already classified as irritant at 0.8% with the “immature” epithelium model, whereas a higher dose was required to obtain the same classification with the standard epidermis model (classified as non-irritant at 0.8% and irritant at 3%). Positive control SDS at 0.5% strongly affected the cellular viability of both epithelia, suggesting a saturation of the experimental models at the selected dosage.
The higher “immature” model sensitivity was consistent with the different morphology of the corneal layer observed on the negative control. As illustrated in figure 7, the “immature” epithelium model was characterized by a thinner stratum corneum expected to reduce the barrier function efficacy.
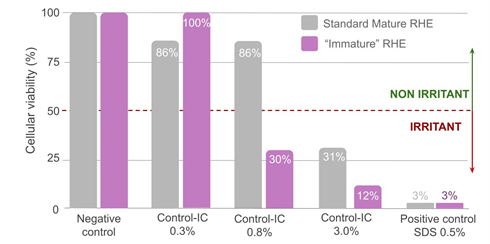
Figure 6: Compared cellular viability of the controls on mature and “immature” reconstructed epithelia.
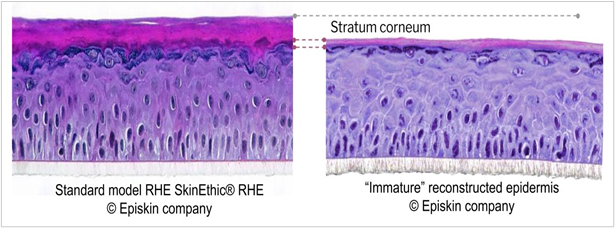
Figure 7: Compared morphology of mature and immature reconstructed epithelia (negative control); Histological section, H&E staining.
- Ingredients and benchmark tolerance
Starting with the effect of positive controls on the other parameters than cell viability shown in table 6, the absence of IL-1∝ release and TEER variation as well as the histology score identical to the negative control confirmed the good tolerance of control-IC at the lowest dose of 0.3%. When looking at the cell viability, control-IC at the doses of 0.8% and 3% showed a poor tolerance as well as the positive control SDS at 0.5% associated with a strong release of pro-inflammatory marker IL-1∝, previously discussed (figure 6).
Benchmarks fully dedicated to babies did not significantly affect cell viability according to the adopted threshold, but provided diverse results on the other parameters, according to the formulation type. O/W emulsions Benchmarks 4 and 5, containing surfactants, both induced released IL-1α concentrations slightly higher than those observed with the negative control (10 and 17 respectively), indicating the initiation of an inflammatory process. Additionally, marked alterations for both emulsions were observed in the immature epithelium structure, cumulating picnosis in the basal layer and perinuclear vacuolization in the basal and in the granular layer (Histology score 12 and 10 respectively). A tendency to decreased cell viability was observed for Benchmark 5 (75%), whereas no significant effect was noticed with the same formula tested on the standard mature RHE epithelium (cell viability= 96%; data not shown). On the contrary, oil-based formula Benchmarks 6 and 7, without any surfactant in their composition, demonstrated a good tolerance, consistent across all parameters, with values not significantly different from the negative control. Beyond the results obtained with the positive SDS control, the sensitivity of 3D reconstructed epithelia to surfactants is well known, as illustrated by the acceptance of correlations based on a lower dose on the in vitro model than the one tested for the patch test48.
These results on well-accepted market products confirmed the high sensitivity of the epithelium and indicated the promising ability of this test to detect early signs when combined with a multiparametric analysis.
None of the ingredients tested affected the cell viability according to the adopted threshold or induced a significant increase of released IL1-∝ compared to the negative control. The moisturizing active ingredient and the rheology modifiers R.M.3 were considered well tolerated at the tested dosage as demonstrated by the association with absence of significant modification of the epidermis structure compared to the negative control. Emulsifier 1 tested at 3%, Emulsifier 2 tested at 2% and rheology modifier R.M. 2 tested at 2% showed a tendency to impair the 3D tissular structure and cellular integrity of the epidermis in the three layers as illustrated by their histology score and led to a conclusion of “acceptable tolerance” relative to the results obtained with Benchmarks 4 and 5. In a conservative approach, complementary trials were performed with a reduced dose of Emulsifier 1 to 2% and R.M. 2 to 1% confirming their non-significant effect on the “immature” epithelium structure. These results clearly demonstrated the interest of adding a histology score to improve the sensitivity of the model compared to investigations based only on cellular viability.
Preliminary trials were also conducted with simple ingredient combinations of Emulsifier 1, Caprylic/Capric triglyceride or the more recent renewable alkane, R.M.1, with and without the moisturizing active ingredient to formulate O/W emulsions. All these trials resulted in the same epithelium structure disturbance as the O/W emulsions Benchmarks 4 and 5, as illustrated by similar high histology scores. Moreover, the control cream gel with R.M.1** and Caprylic/Capric triglyceride, without emulsifier, confirmed the absence of effect. These results and the dose-effect on emulsifier 1 and 2 reinforced the assumption that the model is especially sensitive to surfactant structures, which is probably related to the low thickness of the corneal layer.
The reason for the high histology score induced by R.M. 2 at the highest dose of 2% will need further investigation.
Table 6: Results with “Immature” epidermis model
|
Products |
Dose (wt%) |
Cellular Viability (%) |
IL-1alpha (pg/mL) |
Histological Score (/15) |
Conclusion |
|
|
Controls |
Negative control PBS |
|
100 |
1 |
3 |
Good tolerance |
|
Positive control SDS |
0.5a |
5 (mean; n=3) |
256 (mean; n=3 |
Not Tested |
Poor tolerance |
|
|
Control-IC |
0.3a |
100 |
1.5 |
3 |
Good tolerance |
|
|
0.8a |
30 |
Not Tested |
Not Tested |
Poor tolerance |
||
|
3a |
12 |
Not Tested |
Not Tested |
Poor tolerance |
||
|
Benchmarks |
Benchmark 4 |
Pure |
98 |
10 |
12 |
Acceptable tolerance |
|
Benchmark 5 |
Pure |
75 |
17 |
10 |
Acceptable tolerance |
|
|
Benchmark 6 |
Pure |
100 |
0 |
3 |
Good tolerance |
|
|
Benchmark 7 |
Pure |
100 |
0 |
3 |
Good tolerance |
|
|
Ingredients |
Moisturizer |
5a |
97 |
3 |
3 |
Good tolerance |
|
Emulsifier 1 |
2a |
97 |
Not Tested |
5 |
Good tolerance |
|
|
3a |
97 |
3 |
13 |
Acceptable tolerance |
||
|
Emulsifier 2 |
2a |
100 |
4 |
9 |
Acceptable tolerance |
|
|
R.M. 1 |
2a |
96 |
1 |
4 |
Good tolerance |
|
|
2b |
87 |
0 |
5 |
Good tolerance |
||
|
R.M. 2 |
1a |
100 |
0 |
4 |
Good tolerance |
|
|
2a |
100 |
0 |
11 |
Acceptable tolerance |
||
|
R.M. 3 |
2a |
94 |
3 |
Not tested |
Expected Good tolerance |
|
|
3a |
100 |
3 |
4 |
Good tolerance |
||
|
Ingredients combinations |
Emulsifier 1 + R.M.1 + C8-C10 Triglyceride |
2 + 0.5 + 10a |
95 |
1 |
11 |
Acceptable tolerance |
|
Emulsifier 1 + R.M.1 + C8-C10 Triglyceride + Moisturizer |
2 +1.5 + 10 +3a |
100 |
0.7 |
9 |
Acceptable tolerance |
|
|
Emulsifier 1 + R.M.1 + C15-19 renewable alkane + Moisturizer |
2 +1.5 + 10 +3a |
100 |
1.9 |
11 |
Acceptable tolerance |
|
aIn demineralized water at pH≅6.3-7, bIn C8-C10 Triglyceride 10% + water
Results on experimental reconstructed “impaired” epidermis:
- Validation of model sensitivity versus standard Reconstructed Epidermis (RHE)
Higher sensitivity induced by the controlled abrasion procedure is clearly shown with Control-IC resulting in a non-irritant conclusion at 0.8% with standard RHE compared to irritant conclusion with the “impaired” model (figure 8). For Sodium Dodecyl Sulfate, based on preliminary dose-effect experiments at 1%, 0.5% (data not shown), 0.25% and 0.15%, a 0.15% dose was selected in order to avoid total tissue destruction and be able to measure the other parameters. At this dosage, SDS did not affect viability compared to standard RHE.
The morphology of impaired RHE (negative control), the histology analysis showed a removal of upper corneal layers as well as reduced cohesion (figure 8 - score 0 to 1 shown in table 7), both expected to affect barrier function efficacy compared to the standard RHE (score=0; data not shown) associated with a significant decrease in TEER (see table 7). These observations are consistent with increasing caffeine permeation reported 1 hour after abrasion in another study using the same impaired model36.
The limited viability reduction induced by mechanical abrasion from 100% to 79% on the negative control indicated that the applied damage occurred at the stratum corneum and granular layer level, targeting the epidermal barrier without significantly affecting the viable layers (figure 9). This last point was confirmed by the low migration of biotin in the granular layer (see figure 10).
These results converge towards a greater sensitivity of the “impaired” reconstructed model.
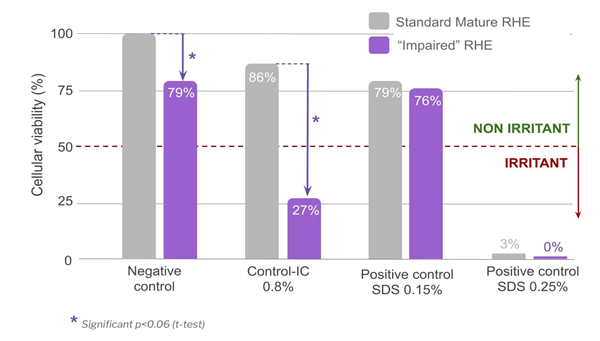
Figure 8: Compared cellular viability of the controls on mature and “impaired” reconstructed epithelia
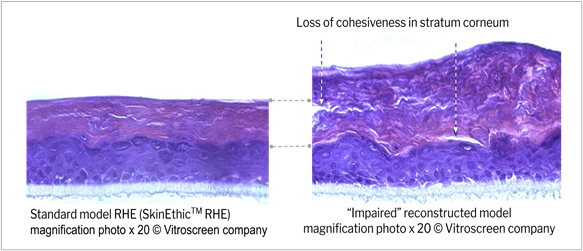
Figure 9: Compared morphology of standard and impaired reconstructed epithelia (negative control) Histological section, H&E staining.
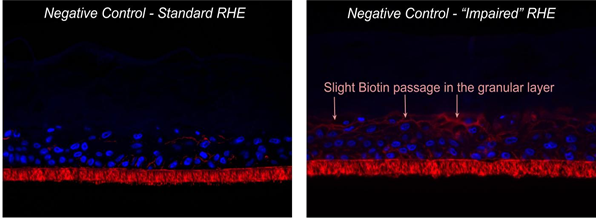
Figure 10: Compared biotin assay in standard and impaired reconstructed epithelia (negative control);
Histological section, DAPI & biotin staining; ⬠biotin migration front.
- Ingredients and benchmark tolerance
The results obtained with the positive controls (SDS and Control-IC) on parameters other than cell viability (table 7) confirmed severe impairment of the epithelial barrier as shown by the strong TEER reduction, associated with marked morphological damage up to the basal layers (including tissue detachment from the polycarbonate) and biotin migration within the whole tissue (shown in figure 13). All these factors pointed to reduced tissue integrity and cell to cell cohesion.
Benchmark 5, a medical device dedicated to skin ulcerations, did not affect cell viability, but generated some structural modifications in the basal and granular layers, as well as strong biotin permeability (TEER increase was non-significant: t-test p value > 0.4, compared to basal value). These results definitively confirmed the high sensitivity of the model, which was one of the objectives in order to maximize the effects of the ingredients.
Table 7:Results with “Impaired” epidermis model
|
Products |
Dose (wt%) |
Cellular Viability (%) |
H&E score |
TEER â% versus t0 |
Biotin permeation |
Conclusion |
|
|
Controls |
Impaired negative control PBS |
|
100 |
1 |
-36/-46/-12 |
No |
Good tolerance |
|
Positive control SDS |
0.25* |
0 |
4 |
-99 |
Yes |
Poor tolerance |
|
|
0.15* |
50 (mean; n=2) |
2.5 (mean; n=2) |
-92 (mean; n=2) |
Yes |
Poor tolerance |
||
|
Control-IC |
0.8* |
34 |
3 |
-87 |
Yes |
Poor tolerance |
|
|
Benchmark |
Benchmark 5 |
Pure |
97 |
2 |
+9 |
Yes |
Acceptable tolerance |
|
Ingredients |
Moisturizer |
5* |
100 |
0-1 |
0 restored structure & morphology |
No |
Good tolerance |
|
Skin repair active |
1*** |
96 |
1 |
+11 |
No lower signal |
Good tolerance |
|
|
Soothing active |
0.2* |
97 |
1 |
+65 |
No lower signal |
Good tolerance |
|
|
Emulsifier 1 |
2* |
100 |
1 |
-38 |
No |
Good tolerance |
|
|
Emulsifier 2 |
2* |
100 |
1 |
-13 |
No |
Good tolerance |
|
|
R.M.1 |
2* |
99 |
1 |
-35 |
No |
Good tolerance |
|
|
R.M. 2 |
1* |
99 |
1 |
-46 |
No |
Good tolerance |
|
|
R.M. 3 |
2* |
92 |
1 |
+55 |
No lower signal |
Good tolerance |
|
|
3* |
90 |
0-1 |
-72 |
No no signal |
Acceptable tolerance |
||
Vehicle: *Demineralized water at pH≅6.3-7, *** Paraffin oil
The high cell viability observed for all the tested ingredients reflects the absence of toxic effects in the viable epidermis. Despite a statistically overall barrier impairment versus t0 (before impairment), Emulsifiers 1 and 2, as well as R.M. 1 and R.M. 2, were not considered different from the negative control. Their good tolerance is consistent with the absence of significant effect on tissue morphology and biotin permeability compared to the negative control.
Application of the three active ingredients and R.M. 3 at 2% induced some interesting signs of tissue recovery. The moisturizing active ingredient provided a tendency to increase in TEER (not statistically significant), but histology analysis revealed an increase of stratum corneum thickness without any modification in the viable layers compared to the negative control, indicating that the product was able to fully recover the barrier modifications induced by the impairment (figure 11). These results are related to the clinically proven restructuring effect of the moisturizing active ingredient tested49. It clearly illustrates the complementarity between local tolerance and efficacy evaluation, and could open prospects for the development of a pre-clinical shared model with adequate multiparametric analysis. Vegetable squalane content in R.M. 3 could be at the root of the observed recovery phenomenon, as it has been well known for decades for its protective effect on skin. Some authors have also reported some defensive effect of squalane against chemically-induced injury in human keratinocytes, as well as slight anti-wrinkle action in a 3D human skin tissue model50. The fact that R.M. 1 based on the same polymeric structure but without squalane in its composition did not show any recovery signs supported this hypothesis, which will need to be further investigated. The skin repair active ingredient seemed to help restore the barrier function despite a non-statistically significant TEER increase compared to the basal value (t-test p value >0.4). Indeed, a statistically significant TEER increase +149% (t-test p value < 0.05) was observed compared to the TEER value recorded after impairment (figure 12), in line with lower biotin permeability compared to impaired negative control (figure 13). The soothing active ingredient and R.M. 3 at 2% both induced a statistically significant TEER increase (t-test p value < 0.01) compared to both the basal and “after impairment” values (+186% and +117% respectively; figure 12) concomitant with a lower biotin permeability compared to the impaired negative control (figure 13). Unexplained isolated TEER effect was observed with R.M. 3 at the higher dosage of 3%. Further dose effects should be investigated to support tolerance level at high doses.
Multiparametric analysis appeared suitable to evaluate the local tolerance of ingredients on impaired epidermis. Moreover, histology, TEER and Biotin permeation assay provided some preliminary data on the mode of action.
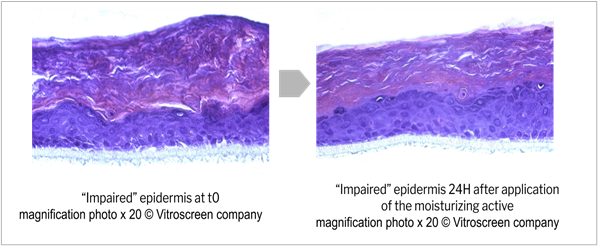
Figure 11: Potential epidermis morphological restoration after application of a moisturizing active; Histological section, H&E staining.
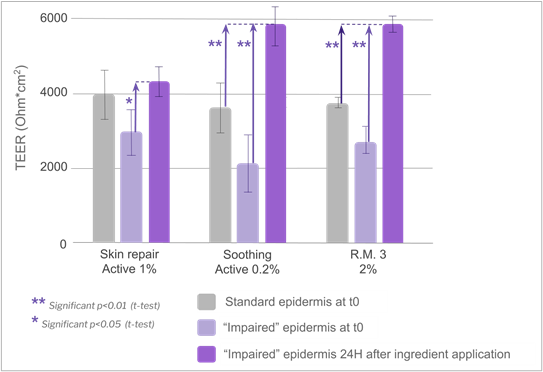
Figure 12. Evolution of TEER after application of specific ingredients.
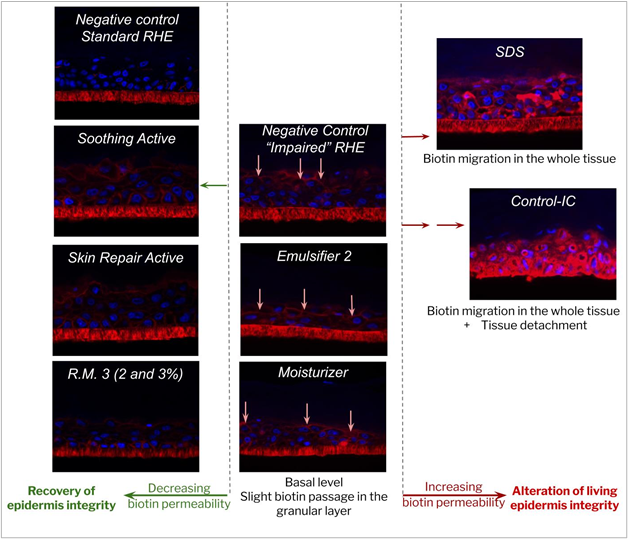
p class="help-block">Figure 13. Examples of the influence of ingredients on biotin migration; Histological section, DAPI & biotin staining; â¬biotin migration front.
Discussion of the Approach and Sensitivity of Models
Two types of approach were implemented in this study. The first one, developed on the two existing reconstructed mucosa models, was based on the assessment of cell viability as a unique read-out parameter of the irritation potential of ingredients. In this first approach, different times of contact with the ingredient, from 10 minutes to 24 hours, were used to modulate the response sensitivity in five categories, from “non irritant” to “very irritant”. Despite the simple analysis, specific answers could be obtained as illustrated by the effect of SDS as a function of time in figure 14 (unusual time scale chosen for better visualization). Vaginal reconstructed epithelium showed higher sensitivity to this surfactant compared to the gingival model, consistent with its predominantly non-keratinized looser structure and its lower impermeable intercellular lipid envelope17. With the aim of replicating the conditions of use as closely as possible, the protocols and in particular the exposure duration were adapted accordingly. 24 hours of contact seems unnecessary for rinse-off ingredients and could probably be shortened. Another option for rinse-off conditions could be to adjust the tested dosage based on a suitable retention factor. Conversely, for some care formulations, especially when mucoadhesion is required for drug delivery purposes, a long contact time is particularly important, and this should be considered for rheology modifiers in general.
This first experience with different ingredient categories also demonstrated that cell viability alone was not sufficient to conclude on local tolerance when the results were poorly reproducible or close to the threshold. This was true, for example, for skin repair active ingredients applied on gingival epithelium. Nevertheless, these first two tests make it possible to compare the ingredients to each other and to give a first indication of the tolerance on this type of mucosa.
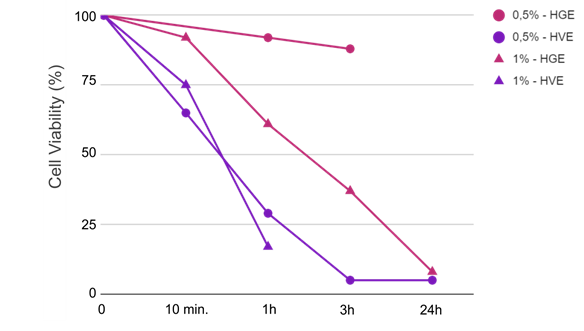
Effect of SDS positive controls on Cell viability; Reconstructed mucosal models.
The second approach, developed to assess local tolerance on “immature” and ”impaired” epidermis, was based on a multiparametric analysis. In this case, the cell viability assessment was limited to a maximized time of contact (16 and 24 h respectively) and additional parameters, focusing on inflammatory response or reflecting tissue structure integrity and barrier functionality, were followed. The results on each model evidenced the input of the multiparametric analysis on the interpretation of results and the reliability of local tolerance conclusions.
It is difficult to compare the sensitivity/prediction of the models, because the chosen parameters were not the same; a great deal of work is still required to optimize the protocols. The choice of parameters was tailored to each specific target in order to improve the predictivity of the model. For babies, due to special susceptibility to irritants, it is particularly important to detect any local discomfort phenomena preceding irritation. It was therefore decided to measure the inflammatory response in the immature model as an early signal. Regarding impaired skins, the monitoring of the barrier function is of great importance because any adverse effects of a formulation would slow down skin recovery and therefore maintain the susceptibility to irritants.
Some preliminary trials on reconstructed “immature” epidermis suggest the possibility to use the model to study the local tolerance of ingredient combinations. The influence of ingredient chemical structures and their interactions should be investigated more deeply and also explored in the other reconstructed models.
Looking at the results from the perspective of ingredient safety, even if it should be considered only as a screening, the global approach is useful to provide guidelines to formulators, according to different situations. Some ingredients, such as the moisturizer, were well tolerated in all the models at the tested concentration, and so can be used on different body areas and in a global formulation chassis. For other structures such as emulsifiers, infra-clinical phenomena (i.e. structural effects) can be perceived depending on the chemical structure. For instance, it led to adapting the dosage of Emulsifier 2 for baby skin care formulations, while classical recommendations were maintained for the other specific body sites tested.
Conclusion and Perspectives
The results obtained from these four in vitro models confirm the high sensitivity of the epithelia compared to standard reconstructed epithelium and show the value of each model in selecting suitable ingredients and determining appropriate dosage for each specific application. Even if these screening models do not include all the markers and specific physiological features of humans, the initial tolerance data can be integrated into a global safety assessment before conducting clinical trials.
Considering the two methodologies that were successively adopted, firstly based on cellular viability evaluation (for existing models) and, in a second step, including several reading points in the cascade of physiological responses (for the two “immature” and “impaired” experimental models), the second approach was clearly demonstrated as the most robust and predictive. The multiple endpoint analysis tailored to the targeted physiological state of each model enriches the basic irritation information with cellular, morphological and functional effect evaluations. This approach also provides early identification of some infra-clinical reactions and mechanistic information. Drawing on this conclusion, one of the first follow-ups would be to optimize the gingival and vaginal models by choosing some tailored additional measuring points. The non-keratinized structure of vaginal epithelium associated with lower barrier function and great susceptibility to infections would suggest starting with the measurement of inflammatory markers and barrier functionality indicators. Comparison with clinical tests will also help to refine the in vitro test conditions and improve the predictivity.
As suggested by the prospective experiments performed on the “immature” model, further investigation on ingredient combinations and more complex formulas can also be seen as a promising perspective to better understand possible interactions and to support the development of new formulation concepts. The acquisition of large amounts of data could also make it possible to identify relationships between the ingredients structure and their effects.
The 3D reconstructed epithelia models offer broad perspectives to cover other body application sites or specific epithelium conditions. The development of colonized models will enable the study of the impact of ingredients on microbiome or otherwise the influence of microbiome on ingredient tolerance47.
It also gives prospects for improving knowledge about ingredient safety, in particular when combined with new concepts and gestures, i.e. aerosols in skin and sun care, concentrated and solid forms to address water scarcity and other environmental considerations.
Acknowledgments
We thank our partners, especially:
Dominique Ostinet from IDEA Lab for her skills in the use of 3D models and help with the analysis of results,
Marisa Meloni from VitroScreen for passionately sharing her expertise, and her always dynamic collaborative approach to the development of innovative models and strategies.
Declaration of competing interests
All the other authors declare no conflict of interest.
References
- Seite S, Bieber T. Barrier function and microbiotic dysbiosis in atopic dermatitis. Clin. Cosmet. Investig. Dermatology. 2015; 8: 479–483.
- Proksch E, Fölster-Holst R, Jensen J-M. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 2006; 43 (3): 159-169.
- Smuth M, Blunder S, Dubrac S, et al. Epidermal barrier in hereditary ichthyoses, atopic dermatitis, and psoriasis. J Dtsch Dermatol Ges. 2015; 13 (11): 1119-1123.
- Kim HS, Lim SH, Song JY, et al. Skin barrier function recovery after diamond microdermabrasion. J. Dermatol. 2009; 36 (10): 529-533.
- Brok Noresslet L, Serup J, Kezic S, et al. Tattoos and skin barrier function: Measurements of TEWL, stratum corneum conductance and capacitance, pH, and filaggrin. Skin Res Technol. 2019; 25 (3): 382-388.
- Misery L, Jourdan E, Huet F, et al. Sensitive skin in France: a study on prevalence, relationship with age and skin type and impact on quality of life. J. Eur. Acad. Dermatol. Venereol. 2018; 32: 791-795.
- Chen W, Dai R, Li L. The prevalence of selfâdeclared sensitive skin: a systematic review and metaâanalysis. J. Eur. Acad. Dermatol. Venereol. 2020; 0 (0).
- Meloni M, Tornier C, Robert C, et al. A Cath-up Validation Study on Reconstructed Human Epidermis (Skinethic RHE) for Full Replacement of the Draize Skin Irritation Test. Toxicol In Vitro. 2010; 24: 257-266.
- Dermatotoxicology. Seventh edition. New York: CRC Press Taylor & Francis group; 2007: Chap. 4; Anatomical Factors Affecting Barrier Function: 39-44.
- Zhen YX, Suetake T, Tagami H. Number of cell layers of the stratum corneum in normal skin - relationship to the anatomical location on the body, age, sex and physical parameters. Arch. Dermatol. Res. 1999; 291: 555â559.
- Fluhr JW, Darlenski R, Taieb A, et al. Functional skin adaptation in Infancy – almost complete but not fully competent. Exp. Dermatol. 2010; 19: 483–492.
- Fluhr JW, Darlenski R, Lachmann N, et al. Infant epidermal skin physiology: adaptation after birth. Br. J. Dermatol. 2012; 166: 483–490.
- Stamatas GN, Nikolovski J, Luedtke MA, et al. Infant Skin Microstructure Assessed In Vivo Differs from Adult Skin in Organization and at the Cellular Level. Pediatr Dermatol. 2010; 27 (2): 125–131.
- Fluhr JW, Lachmann N, Baudouin C, et al. Development and organization of human stratum corneum after birth: electron microscopy isotropy score and immunocytochemical corneocyte labelling as epidermal maturation’s markers in infancy. Br. J. Dermatol. 2014; 171: 978–986.
- Schlupp P, Weber M, Schmidts T, et al. Development and validation of an alternative disturbed skin model by mechanical abrasion to study drug penetration. Results Pharma Sci. 2014; 4: 26-33.
- Boncheva M. The physical chemistry of the stratum corneum lipids. Int J Cosmet Sci. 2014; 36: 505–515.
- Anderson DJ, Marathe J, Pudney J. The structure of the human vaginal Stratum Corneum and its role in immune defense. Am. J. Reprod. Immunol. 2014; 71: 618–623.
- Dawson DV, Drake DR, Hill JR, et al. Organization, Barrier Function And Antimicrobial Lipids Of The Oral Mucosa. Int J Cosmet Sci. 2013; 35 (3): 220–223.
- Thompson IOC, Van der Bijl P, Van Wyk CW, et al. A comparative light-microscopic, electron-Microscopic and chemical study of human vaginal and buccal epithelium. Arch. Oral Biol. 2001; 46: 1091–1098.
- Van der Bijl P, Van Eyk AD, Thompson IOC. Permeation of 17β-estradiol through human vaginal and buccal mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol. 1998; 85 (4): 393-398.
- Van Der Bijl P, Penkler L, Van Eyk AD. Permeation of Sumatriptan Through Human Vaginal and Buccal Mucosa. Headache. 2000; 40 (2): 137-141.
- Van der Bijl P, Van Eyk AD, Van Wyk CW, et al. Diffusion of reduced arecoline and arecaidine through human vaginal and buccal mucosa. J Oral Maxillofac Pathol. 2001; 30 (4): 200-205.
- Van der Bijl P, Van Eyk AD. Human Vaginal Mucosa as a Model of Buccal Mucosa for In Vitro Permeability Studies: An Overview. Curr Drug Deliv. 2004; 1 (2): 129-135.
- OECD Guideline for the Testing of Chemicals No. 439. In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. 2020. Available from: https://www.oecdilibrary.org.
- Cotovio J, Grandidier MH, Lelièvre D, et al. In vitro Acute Skin Irritancy of Chemicals Using the Validated EPISKIN Model in a Tiered Strategy - Results and Performances with 184 Cosmetic Ingredients. AATEX 14. 2007; 14 (Special Issue): 351-358.
- Spielmann H, Hoffmann S, Liebsch M, et al. The ECVAM International Validation Study on In vitro Tests for Acute Skin Irritation: Report on the Validity of the EPISKIN and EpiDerm Assays and on the Skin Integrity Function Test. ATLA. 2007; 35: 559-601.
- Perkins M.A, Osborne R, Rana F.R, Ghassemi A., Robinson M.K. Comparison of In Vitro And In Vivo Human Skin Responses to Consumer Products and Ingredients with a range of Irritancy Potential. Toxicol. Sci. 1999; 48: 218-229.
- Welss T, Basketter D, Schröder KR. In vitro skin irritation facts and future. State of the art review, mechanism and models. Toxicol In Vitro. 2004; 18: 231-243.
- Catoire S, Lopez F, Mora D, et al. An Immature Human Reconstructed Epidermis Model To Assess Infant Personal Care Product Ranges. Poster session presented at XVI Congreso National de Biotechnologia y Bioingenieria; 2015; Mexico.
- Roden K, Thelu A, Catoire S, et al. Assessing Skin Irritation Potential Of Baby Personal Products Using The Thor In Vitro Baby Reconstructed Human Epidermis (Rhe) Model: The Baby Vitroderm. The Science Of Beauty (Formerly The Australasian Journal Of Cosmetic Science). 2015; 5 (1): 40-47.
- Meloni M, Ceriotti L, Galizia G, et al. Hard Water and Cleansers: An In-Vitro Study of the Effects on Immature Reconstructed Human Epidermis. Dermatology Research. 2019; 1 (1): 1-8.
- Wurzburger L, Kazmi P, Re T, et al. Evaluation of an oral care product safety screening program utilizing the in vitro skinethic human gingival epithelium (RHG) and oral buccal (RHO) models. Poster session presented at the 50th SOT Annual Meeting, In Proceedings; 2011, 6–10 March; Washington, DC, USA.
- Ayehunie S, Cannon C, Lamore S, et al. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol In Vitro. 2006; 20: 689–698.
- Meloni M, De Servi B, Ghioni A. In Vitro Assessment Of Skin And Mucosal Tolerance Of Cosmetic Products. Alternatives To Animal Tests Journal. 2010; Online Supplement 2 (10).
- Alépée N, Leblanc V, Grandidier MH, et al. Development of the SkinEthic HCE Time-to-Toxicity test method for identifying liquid chemicals not requiring classification and labelling and liquids inducing serious eye damage and eye irritation. Toxicol In Vitro. 2020; 69, 104960.
- Balzaretti S, Ceriotti L, Carriero F, et al. Development And Validation Of A Rhe Model With Impaired Barrier Function To Assess Class Iib Medical Device Biocompatibility. Poster session presented at 58th Annual Meeting of the Society of Toxicology; 2019, March 10-14; Baltimore, USA.
- Meloni M, Dalla Valle P, et al. The importance of multiple endpoint analysis (MEA) using reconstituted human tissue models for irritation and biocompatibility assay. Poster session presented at Invitox. Congress Proceedings. 2002, 16-19 October; 4 (7); Formia, Italy.
- Pissavini M, Diffey B, Marguerie S, et al. Predicting the efficacy of sunscreens in vivo veritas. Int J Cosmet Sci. 2012; 34: 44-48
- Weig EA, Tull R, Chung J, et al. Assessing factors affecting sunscreen use and barriers to compliance: a cross-sectional survey-based study. J Dermatol Treat. 2020; 31 (4): 403-405.
- Serup J, Kettis Lindblad Å, et al. To Follow or Not to Follow Dermatological Treatment – A Review of the Literature. Acta Derm Venereol. 2006; 86: 193–197.
- Merat E, Roso A, Sourdon C, et al. Psycho-rheology, Insight On Polymers. The Science Of Beauty. 2017; 6 (4) 42-46.
- Savic S, Vuleta G, Daniels R, et al. Colloidal microstructure of binary systems and model creams stabilized with an alkylpolyglucoside non ionic emulsifier. Colloid Polym Sci. 2005; 283 (4): 439–451.
- Lukic M, Pantelic I, Daniels R, et al. Moisturizing Emulsion Systems Based On The Novel Long Chain Alkyl Polyglucoside Emulsifier. J. Therm. Anal. Calorim. 2013; 111(3): 2045-2057.
- Dumont S, Cattuzzato L, De Pooter A, et al. Effect Of An Active Ingredient On The Expression Of Barrier Function-related Genes And Moisturization-related Proteins. Int J Cosmet Sci. 2012; 63 (4): 286-287.
- Ramis JM, Coelho CC, Córdoba A, et al. Safety Assessment of Nano- Hydroxyapatite as an Oral Care Ingredient according to the EU Cosmetics Regulation. Cosmetics. 2018; 5 (53) doi:10.3390/cosmetics 5030053 Available from: www.mdpi.com/journal/cosmetics.
- Ayehunie S, Cannon C, LaRosa K, et al. Development of an in vitro alternative assay method for vaginal irritation. Toxicology. 2011; 11, 279 (1-3), 130–138.
- Meloni M, Fontaine T, Devastato C, et al. Lactobacillus Sp. Colonized 3d Human Vaginal Mucosa: Application To Assess Mildness And Properties Of Intimate Hygiene Cosmetics. Poster presented at 28Th Ifscc Congress Paris, "Cosmetic Innovation And Performance For Beauty And Well-being"; 2014, 27-30 October; Paris, France.
- Walters RM, Gandolfi L, Mack MC, et al. In Vitro Assessment of Skin Irritation Potential of Surfactant-based Formulations by Using a 3-D Skin Reconstructed Tissue Model and Cytokine Response. 2016. ATLA; 44: 523-532.
- Dumont S, Cattuzzato L, De Pooter A, et al. Effect Of An Active Ingredient On The Expression Of Barrier Function-related Genes And Moisturization-related Proteins. Paper presented at Annual Scientific Seminar Of The Society Of Cosmetic Chemists Scc; 2012, May 31 - June 1; Charleston, South Carolina, Usa.
- Kato S, Aoshima H, Saitoh Y, et al. Defensive effects of fullerene-C60 dissolved in squalane against the 2,4-nonadienal-induced cell injury in human skin keratinocytes HaCaT and wrinkle formation in 3D-human skin tissue model. J Biomed Nanotechnol. 2010; 6(1): 52-8.
