Cryptococcal Cellulitis in a Patient Immunocompromised Secondary to Cirrhosis
Dillon D Clarey, Adam V Sutton, Ryan M Trowbridge*
University of Nebraska Medical Center, Department of Dermatology, Omaha, NE
Abstract
Cryptococcal cellulitis is a rare dermatologic diagnosis most often seen in immunocompromised patients. We present a case of disseminated Cryptococcus presenting as cellulitis in a patient with decompensated cirrhosis. We discuss various presentations of Cryptococcus in the skin, both as primary and disseminated disease.
Introduction
Cryptococcus is an encapsulated yeast of which five serotypes have been identified to date. Var neoformans, more common in immunocompromised individuals, has been isolated from bird feces, soil, plants, and contaminated foods, while var gattii, more common in immunocompetent individuals, is mostly located in tropical and subtropical regions in association with eucalyptus trees1. Causes of an immunocompromised state associated with cryptococcal infection include immunosuppressive medications, malignancy, HIV, and solid organ transplant, among others1.
An individual becomes infected by Cryptococcus after inhalation into the lungs3. Roughly one-third of immunocompetent patients who develop pulmonary cryptococcal infections will be asymptomatic, while roughly half will have chest pain and cough4. The extrapulmonary findings of Cryptococcus are most commonly seen after hematogenous spread from the lungs to other organs (central nervous system (CNS), bone, skin), with roughly 10-20% of disseminated cases showing skin involvement5. Of note, cutaneous Cryptococcus may precede systemic symptoms by as long as 8 months6. Rarely, an individual can acquire primary cutaneous cryptococcosis infection secondary to direct inoculation from trauma to the skin, with no evidence of disseminated infection7.
The objective of this paper is to report a case of disseminated cutaneous cryptococcal infection in a patient with decompensated cirrhosis.
Case Report
A 64-year-old male with past medical history of ulcerative colitis, decompensated cirrhosis secondary to primary sclerosing cholangitis, hepatocellular carcinoma status-post chemoembolization (on list for liver transplant, MELD-Na 22), and hypertension was admitted to the hospital with a 3-week history of a painful and warm erythematous plaques on his bilateral thighs. The plaques were expanding in size despite one week of cephalexin. Notable medications included mesalamine, lactulose, rifaximin, and spironolactone. Upon admission, labs revealed a white blood cell (WBC) count of 5.5 x 103/uL, platelets 84 x 103/uL, and INR 1.4. A CT of the chest/abdomen/pelvis was significant for new patchy ground-glass and nodular opacities in the upper lungs. A duplex ultrasound (US) of the lower extremity deep venous system was negative for deep venous thrombosis. The patient was admitted for presumed cellulitis and started on intravenous vancomycin. Dermatology was consulted to further evaluate the patient.
On dermatologic examination, the patient had ill-defined, indurated, erythematous plaques on the bilateral medial thighs (Figure 1a, Figure 1b). Differential diagnosis included superficial thrombophlebitis, cellulitis, eosinophilic cellulitis, and cryptococcal cellulitis. Duplex US was negative for superficial thrombophlebitis. Given the bilateral nature of the plaques and induration on exam, along with the immunosuppressed status of the patient and upper lung ground glass opacities, a more extensive work-up was performed along with a punch biopsy. Serum cryptococcal antigen was obtained and returned at 1:320, significantly raising concern for a cryptococcal infection. Due to the propensity of Cryptococcus to disseminate to the CNS, a lumbar puncture was performed. Cerebrospinal fluid (CSF) cell count was notable for WBC 14 /mm3 (normal 0-8 /mm3, 42% polymorphonuclears (normal 0-6%), 17% lymphocytes (normal 40-80%)). CSF cryptococcal antigen and fungal culture (1 of 2) were positive for Cryptococcus neoformans.
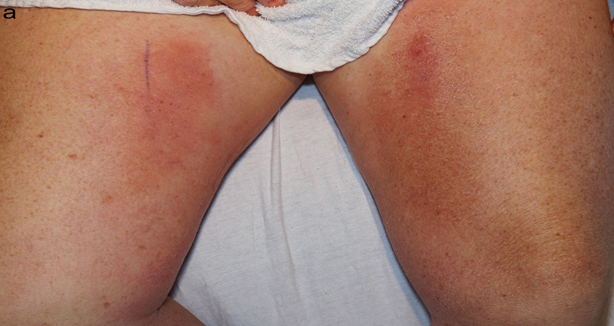
Figure 1(a): Ill-defined, indurated, erythematous plaques on the bilateral medial thighs.
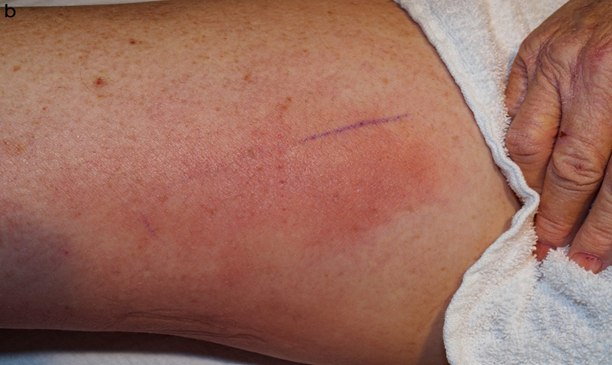
Figure 1(b): Close-up view of erythematous plaque present on the right medial thigh.
Punch biopsy from an erythematous plaque present on the right medial thigh revealed a superficial and deep dermal mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and occasional neutrophils (Figure 2) in addition to numerous yeast-shaped fungal organisms with GMS stain (Figure 3) that also stained positive with mucicarmine (Figure 4). The histologic features were consistent with Cryptococcus neoformans, confirming the diagnosis of disseminated cryptococcal cellulitis and meningitis.
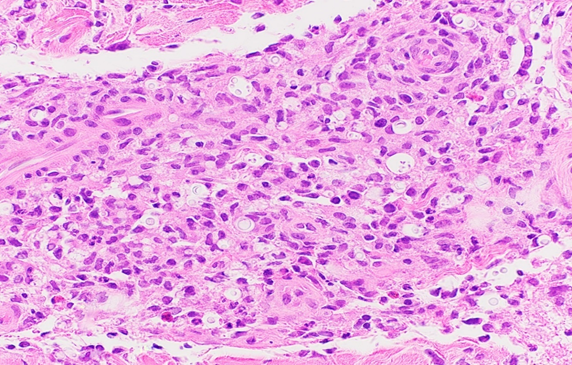
Figure 2: Hematoxylin and eosin (H&E) stain revealing numerous variable sized yeasts contained within clear spaces surrounded by a predominantly mononuclear infiltrate.
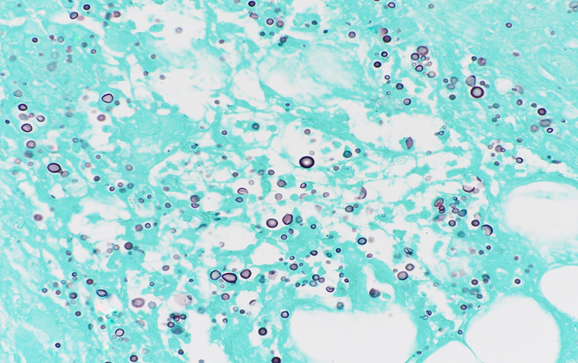
Figure 3: GMS stain showing gray-black reaction with the fungal wall.
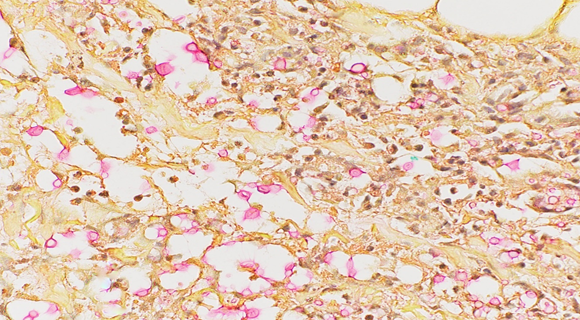
Figure 4: Mucicarmine stain showing pink-to-red staining of the mucinous capsule composed of acid mucopolysaccharides.
The erythema and induration of the plaques on the thighs rapidly improved with clobetasol (for possible contact versus stasis dermatitis) and showed further improvement with amphotericin (5 mg/kg IV every 24 hours) and flucytosine (25 mg/kg IV every 6 hours). Two weeks after admission, the patient developed acute kidney injury likely secondary to amphotericin administration. Pulmonary edema (noted on serial X-rays) developed and lead to acute hypoxic respiratory failure 16 days after admission. Despite diuresis with furosemide, he was admitted to the intensive care unit with acute hypoxic respiratory failure and intubated. Shortly after, the family of the patient opted for comfort measures, and the patient was compassionately extubated. Within hours of extubation, the patient passed away. The cause of death was acute respiratory failure with hypoxia in the setting of pulmonary edema due to acute kidney injury.
Discussion
Cryptococcal cellulitis is a rare diagnosis secondary to primary or disseminated infection. Physical exam findings can help differentiate skin lesions caused by disseminated infection from those caused by primary cutaneous cryptococcosis. Disseminated Cryptococcus appears most commonly as scattered umbilical papules (similar to molluscum contagiosum)7, while primary cutaneous cryptococcosis skin lesions are usually found on unclothed areas (exposed to trauma/inoculation), are solitary, and most often resemble cellulitis7. Primary cutaneous cryptococcosis has been associated with a wide variety of lesions, including papules, nodules, pustules, abscesses, subcutaneous swellings, and ulceration. Cutaneous cryptococcal infection should be on the differential for cellulitis in atypical locations, containing ulceration, or unresponsive to treatment. In this case, the bilateral nature of the erythematous plaques made a diagnosis of cellulitis unlikely and led the dermatology consult team to seek alternative diagnoses.
In the above case, immunosuppression was multifactorial. Decompensated cirrhosis was the most likely contributing factor to immunosuppression. Individuals with decompensated liver disease have a 24-fold increase in risk of cryptococcemia and cryptococcal meningitis and make up 21-36% of cases of Cryptococcus in non-HIV patients8-12. Cirrhosis results in immunosuppression due to defects in the host immune response, including phagocyte dysfunction, decreased antibody and immunoglobulin production, complement deficiency, and impaired cell-mediated immunity8-11. The hypothesis of pulmonary Cryptococcus reactivation from immunosuppression is supported by the fact that the majority of liver transplant patients have cryptococcal antibodies prior to transplant3,13. Mesalamine’s mechanism is poorly understood, although it likely has anti-inflammatory effects (reduction of pro-inflammatory prostaglandins and leukotrienes)14. However, mesalamine usage showed no association with increased infection (odds ratio 1.0, p = 0.94) in one study of patients with ulcerative colitis and Crohn’s disease15. Although this patient had a history of hepatocellular carcinoma, it was treated roughly one-year prior with chemoembolization, thus this was likely noncontributory to the immunosuppressed state. Lastly, ulcerative colitis and primary sclerosing cholangitis are more often associated with immune system activation16,17.
Management of cryptococcal infection varies upon disease severity, underlying immune status and comorbid conditions (HIV, organ transplant history), and presence or absence of dissemination, with medication regimens including induction, consolidation, and maintenance therapies18. Despite defined treatment algorithms, disseminated Cryptococcus has been associated with high mortality rates (63% in one case series of 30 HIV-uninfected patients with disseminated disease (mean survival 21 days))19.
In this case, we report a patient with cirrhosis, ulcerative colitis, and primary sclerosing cholangitis who was diagnosed with disseminated (CSF, serum, skin) cryptococcal infection and subsequently passed from disease management complications. Early recognition of cellulitis not responding to conventional management, especially in immunosuppressed patients, should point the clinician to consider revisiting the diagnosis. This is key in diagnosis of Cryptococcus.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Dora JM, Kelbert S, Deutschendorf C, et al. Cutaneous cryptococccosis due to Cryptococcus gattii in immunocompetent hosts: case report and review. Mycopathologia. 2006; 161(4): 235-238.
- Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc. http://www.ncbi.nlm.nih.gov/pubmed/23874010%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3715903. Published 2013. Accessed August 5, 2019.
- Saha DC, Goldman DL, Shao X, et al. Serologic Evidence for Reactivation of Cryptococcosis in Solid-Organ Transplant Recipients. Clin Vaccine Immunol. 2007; 14(12): 1550-1554.
- Campbell GD. Primary pulmonary cryptococcosis. Am Rev Respir Dis. 1966; 94(2): 236-243.
- Biancheri D, Kanitakis J, Bienvenu AL, et al. Cutaneous cryptococcosis in solid organ transplant recipients: epidemiological, clinical, diagnostic and therapeutic features. Eur J Dermatology. 2012; 22(5): 651-657.
- Moorani KN, Khan KMA, Ramzan A. Infections in children with nephrotic syndrome. J Coll Physicians Surg Pak. 2003; 13(6): 337-339.
- Neuville S, Dromer F, Morin O, et al. Primary Cutaneous Cryptococcosis: A Distinct Clinical Entity. Clin Infect Dis. 2003; 36(3): 337-347.
- Chuang Y, Ho Y, Chang H, et al. Disseminated cryptococcosis in HIV-uninfected patients. Eur J Clin Microbiol Infect Dis. 2008; 27(4): 307-310.
- Hwang SY, Yu SJ, Lee JH, et al. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis. 2014; 33(2): 259-264.
- Singh DK, Tyagi I, Saran RK, et al. Fatal spontaneous Cryptococcal peritonitis in a woman with decompensated liver cirrhosis. Acta Cytol. 2010; 54(5 Suppl): 1087-1089. http://www.ncbi.nlm.nih.gov/pubmed/21053617. Accessed August 5, 2019.
- Singh N, Husain S, de Vera M, et al. Cryptococcus neoformans Infection in Patients With Cirrhosis, Including Liver Transplant Candidates. Medicine Baltimore. 2004; 83(3): 188-192.
- Lin Y, Shiau S, Fang C. Risk Factors for Invasive Cryptococcus neoformans Diseases A Case-Control Study. Nielsen K ed. PLoS One. 2015; 10(3): e0119090.
- Singh N, Sifri CD, Silveira FP, et al. Unique characteristics of cryptococcosis identified after death in patients with liver cirrhosis: comparison with concurrent cohort diagnosed antemortem. Med Mycol. 2017; 55(3): 278-284.
- Ye B. Mesalazine preparations for the treatment of ulcerative colitis: Are all created equal? World J Gastrointest Pharmacol Ther. 2015; 6(4): 137.
- Toruner M, Loftus E V, Harmsen WS, et al. Risk Factors for Opportunistic Infections in Patients With Inflammatory Bowel Disease. Gastroenterology. 2008; 134(4): 929-936.
- Aron JH, Bowlus CL. The immunobiology of primary sclerosing cholangitis. Semin Immunopathol. 2009; 31(3): 383-397.
- Wallace KL, Zheng LB, Kanazawa Y, et al. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014; 20(1): 6-21.
- Perfect JR, Dismukes WE, Dromer F, et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2010; 50(3): 291-322.
- Chuang Y, Ho Y, Chang H, et al. Disseminated cryptococcosis in HIV-uninfected patients. Eur J Clin Microbiol Infect Dis. 2008; 27(4): 307-310.
