A Very Rare Case of Immunoglobulin A Vasculitis in an Adult with Alcoholic Liver Cirrhosis
Kanthi Bommareddy1, Rabah Alreshq1, David Jones2, Gurpreet Singh1*
1Department of Medicine, Albany Medical Center, Albany, NY 12208
2Department of Pathology and Laboratory Medicine, Albany Medical Center, Albany, NY 12208
Abstract
Immunoglobulin A vasculitis, formerly known as Henoch-Schönlein purpura, is an IgA-mediated small-vessel vasculitis that predominantly affects children with an incidence of 10-20 per 100,000 per year. It is very unusual in adults; however, cirrhosis has been associated with immunoglobulin A vasculitis because of the cirrhotic liver’s inability to metabolize circulating IgA complexes, resulting in systemic deposition particularly in the skin and kidney. In cirrhosis, the most common causes of acute kidney injury are those of prerenal azotemia including hepatorenal syndrome followed by intrarenal causes. Our very rare case of kidney injury in a cirrhotic patient is due to deposition of circulating IgA complexes. We present a very rare case of palpable purpura and acute kidney injury consistent with immunoglobulin A vasculitis in an adult with alcoholic cirrhosis. This patient’s skin and renal findings improved with oral prednisone.
Case Presentation
A 56-year-old male presented with a two-week history of bilateral lower limb swelling with scattered pruritic, palpable purpura. He denies fever, upper respiratory symptoms or other infectious symptoms. Past medical history was significant for alcoholic liver cirrhosis and a two-month history of asymptomatic rise in creatinine from 0.76 mg/dL to 1.84 mg/dL. The patient denies recent illness, allergic reaction, or change in medication. Physical examination of the lower extremities revealed numerous 5 mm non-blanching, palpable purpura with 3+ pitting edema. Abdomen was soft, distended with a positive fluid wave, and non-tender to palpation. Complete blood count revealed thrombocytopenia (30,000 platelets/µL), and complete metabolic panel revealed creatinine of 2.23 mg/dL and estimated glomerular filtration rate (eGFR) of 31 mL/min/1.73 m2, measured by MDRD study equation (Modification of Diet in Renal Disease).
Diagnosis & Treatment
Laboratory values showed deteriorating renal function. 24-hour urine protein showed nephrotic range proteinuria (3328 mg/24 hour), and urinalysis indicated 3+ hemoglobin with numerous red blood cells with red blood cell casts. Given the purpuric rash and hematuria with proteinuria, vasculitis was high on the differential diagnosis. Nephrology was consulted, but renal biopsy was deferred because of the risk of bleeding from thrombocytopenia.
Hepatitis B surface antigen, hepatitis C virus polymerase chain reaction, hepatitis C virus antibody, and human immunodeficiency virus antibody were all negative. ANA, anti-dsDNA, anti-MPO, and anti-PR3 were negative. The serum C3 level was 101.2 (normal 87-200) mg/dL and serum C4 level was 22 (normal 19-52) mg/dL.
Punch biopsy of the purpura showed extravasated erythrocytes, polymorphonuclear cells, and nuclear debris surrounding microvasculature in the dermis with sparse superficial perivascular lymphocytic infiltrate. Immunofluorescence revealed granular deposits of C3, C5b-9, C4D, IgM, and IgA with no IgG (Figure 1).
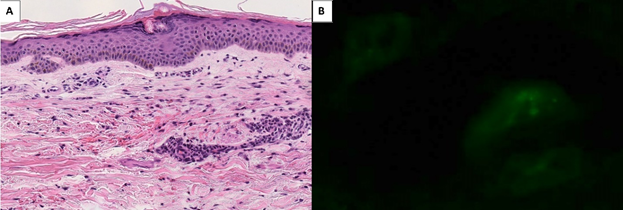
Figure 1: Punch biopsy of 56-year old male with purpura. Biopsy specimen shows mononuclear infiltrate and inflammatory debris surround small caliber vessels (panel A). There is perivascular extravasated red blood cells and fibrin deposition (H&E, original magnification x200). Immunofluorescence revealed granular deposits of IgA with no IgG deposition circumferentially around dermal blood vessels (panel B).
The latest criteria for diagnosing immunoglobulin A vasculitis in adults is the European League Against Rheumatism/Paediatric Rheumatology International Trials Organisation/Paediatric Rheumatology European Society (EULAR/PRINTO/PRES). This criterion has a sensitivity of 99.2% and specificity of 96% in adults1.
This criterion includes purpura or petechiae and one of the following four:
1. Abdominal pain.
2. Arthritis/Arthralgia.
3. Renal involvement.
4. Leukocytoclastic vasculitis with predominant IgA deposits or proliferative glomerulonephritis with predominant IgA deposits.
Our patient’s purpura, renal involvement, and skin biopsy meet the diagnosis of immunoglobulin A vasculitis. The patient was started on prednisone 60 mg oral tablet daily, and his rash resolved within two weeks. At two weeks, his creatinine decreased to 1.84 mg/dL, and his eGFR rose to 38 mL/min/1.73 m2. After 7 months’ post-discharge, his creatinine decreased to 0.83 mg/dL, and his eGFR rose to >60 mL/min/1.73 m2. Prerenal differentials like hepatorenal syndrome are less likely because of his skin findings and improvement in kidney function with prednisone.
Discussion
Cirrhosis prevalence is approximately 630,000 people in the United States2 and is expected to rise because of rising 5-year survival rates. Prerenal azotemia like hepatorenal syndrome is high on the differential for acute kidney injury in cirrhosis. Intrarenal causes like immunoglobulin A vasculitis is a rare manifestation of cirrhosis and has been reported in only thirteen cases. Each case presented with varying levels of dermal, renal, and gastrointestinal involvement (Table 1). The causes of cirrhosis in twelve cases include viral hepatitis, primary biliary cirrhosis, alcohol, and medication use. Cirrhosis leads to decreased clearance of serum IgA and results in dermal, renal mesangial and gastrointestinal IgA deposition3. Coexisting cirrhosis and IgA glomerular deposition can be unrelated; however, the incidence of glomerular IgA deposition is up to 50%-100%, arguing for a possible association between the two diseases4. In addition, animal models show liver injury leads to increased serum IgA, IgA immune complexes, and IgA deposition in mesangial deposition5. To date there are no treatment guidelines for adults with immunoglobulin A vasculitis.
The differential for rash in our patient includes thrombocytopenic petechiae, immunoglobulin A vasculitis, or cryoglobulinemia. Biopsy showed dermal extra-capillary IgA deposition consistent with immunoglobulin A vasculitis. Van de Wiel et. al. reported 68% of cirrhosis patients presented with immune complex deposition in the dermis; however, very few presented with purpura. 87% of those with measurable IgA-containing immune complexes (CIC) in serum demonstrated IgA skin deposits, whereas 53% of those without IgA CICs in serum had IgA skin deposits6. Of the thirteen reported cases of immunoglobulin A vasculitis in cirrhosis patients in literature, twelve cases presented with a purpura and one presented with a non-descript skin rash. Patients often seek medical care because of acute skin changes since many other symptoms of immunoglobulin A vasculitis, including hematuria, proteinuria, and abdominal pain, can be missed, misattributed to cirrhosis, or non-descript.
Hepatic disease is associated with glomerular lesions, most commonly membranous nephropathy, crescentic glomerulonephritis, proliferative and exudative glomerulonephritis, and hepatic IgA glomerulonephritis. Hepatic IgA glomerulonephritis results from IgA deposition; however, it rarely produces nephritic urinary sediment unlike primary or purpuric IgA glomerulonephritis7. Our patient has demonstrated marked hematuria and proteinuria in addition to skin involvement. The severity of IgA nephropathy in immunoglobulin A vasculitis varies widely; however, severity of renal involvement is the most important long-term prognostic factor8. Immunoglobulin A vasculitis nephritis with nephrotic syndrome is more likely to progress to end-stage renal disease and has a worse outcome than immunoglobulin A vasculitis nephritis alone9. Ten cases reported hematuria, proteinuria, or oliguria consistent with immunoglobulin A vasculitis nephropathy. Eight of the ten cases presented with hematuria, seven of which exhibited concomitant nephrotic syndrome. In this patient with proteinuria and hematuria, immunoglobulin A vasculitis caused a prolonged acute kidney injury, one that began three months prior to admission without any respiratory illness and ended seven months after admission.
While this patient did not report gastrointestinal (GI) symptoms, the presence of gastrointestinal disease, including perforation and hemorrhage is the most important determinant of length of hospital admission10. Eight cases presented with GI complaints.
Immunoglobulin A vasculitis is a self-limiting disease with average symptom duration of 4-6 weeks. Early use of glucocorticoids has not been shown to reduce the risk of renal or gastrointestinal involvement; however, use of prednisone has been shown to help resolve abdominal pain and rash in a previous case report11. In patients with cirrhosis and immunoglobulin A vasculitis, five of the eight patients treated with corticosteroids had resolution of symptoms; other patients did not survive because of their decompensated gastrointestinal pathology.
Table 1. Reported cases of immunoglobulin A vasculitis in patients with liver cirrhosis.
|
First Author |
Age |
Sex |
Cause of Cirrhosis |
Presenting Symptoms |
Treatment |
Outcome |
|
Ogawa et. al. (1995)[12] |
56 |
M |
Hepatitis C Virus |
Purpuric rash + proteinuria and hematuria |
Plasmapheresis + corticosteroids |
Deceased |
|
Madison et. al. (2002)[13] |
63 |
M |
Hepatitis C Virus |
Purpuric rash + hematuria + abdominal pain |
Corticosteroids |
Deceased |
|
Akizue et. al. (2017)[14] |
62 |
M |
Hepatitis B Virus |
Purpuric rash + proteinuria and hematuria + abdominal pain |
Corticosteroids |
Resolved |
|
Aggarwal et. al. (1992)[15] |
63 |
F |
Not specified |
Purpuric rash + hematuria, proteinuria, and oliguria |
Hemodialysis |
Unresolved |
|
Fleischman et. al. (2017)[16] |
52 |
M |
Not specified |
Purpuric rash + oliguria and proteinuria + abdominal pain |
Corticosteroids |
Progressive kidney failure |
|
Miret Mas et. al. (2003)[17] |
46 |
M |
Alcohol |
Purpuric rash + proteinuria, hematuria, proteinuria |
Not specified |
Not specified |
|
Jung et. al. (2016)[18] |
54 |
M |
Alcohol |
Skin rash + proteinuria + abdominal pain |
Hemodialysis |
Resolved |
|
Gweon et. al. (2019)[19] |
42 |
M |
Alcohol |
Petechial rash + proteinuria and hematuria + abdominal pain |
Corticosteroids |
Resolved |
|
Gupta et. al. (2015)[3] |
58 |
M |
Alcohol |
Purpuric rash + proteinuria and hematuria + abdominal pain |
Corticosteroids + hemodialysis |
Deceased |
|
Gatselis et. al. (2007)[20] |
71 |
F |
Primary Biliary Cirrhosis |
Purpuric rash + proteinuria and hematuria + abdominal pain |
Corticosteroids |
Resolved |
|
72 |
F |
Primary Biliary Cirrhosis |
Purpuric rash |
None |
Resolved |
|
|
Mu et. al. (2018)[21] |
12 |
M |
Drug-Induced |
Purpuric rash + abdominal pain |
Corticosteroids + umbilical cord-derived Mesenchymal Stem Cell transplant |
Resolved with transplant |
|
Kitamura et. al. (2006)[22] |
66 |
M |
Hepatitis C Virus |
Purpuric rash |
Corticosteroids |
Resolved |
*Robeva, Joerg, Korting, Bolliger, Gaeleone have published work regarding immunoglobulin A vasculitis in cirrhosis patients as well.
Conclusion
Cirrhosis is commonly associated with purpuric rash and usually attributed to thrombocytopenic petechiae. In the presence of renal insufficiency with hematuria and proteinuria, a clinician should consider immunoglobulin A vasculitis in the differential diagnosis even in the absence of any respiratory illness.
Acknowledgements
No financial support for this study. There are no conflicts of interests.
References
- HoÄevar A, Rotar Z, JurÄiÄ V, et al. IgA vasculitis in adults: the performance of the EULAR/PRINTO/PRES classification criteria in adults. Arthritis Res Ther. 2016. 18: 58.
- Scaglione S, Kliethermes S, Cao Guichan, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015. 49(8): 690-6.
- Gupta N, Kim J, and Njei B. Spontaneous Bacterial Peritonitis and Henoch-Schonlein Purpura in a Patient with Liver Cirrhosis. Case Rep Med. 2015. 47(6): 619-21.
- Newell GC. Cirrhotic glomerulonephritis: incidence, morphology, clinical features, and pathogenesis. Am J Kidney Dis. 1987. 9(3): 183-90.
- Nochy D, Druet P, and Bariety J. IgA nephropathy in chronic liver disease. Contrib Nephrol. 1984. 40: 268-75.
- Van de Wiel A, Valentijin RM, Schuurman HJ, et al. Circulating IgA immune complexes and skin IgA deposits in liver disease. Relation to liver histopathology. Dig Dis Sci. 1988. 33(6): 679-84.
- Nakamoto Y, Lida H, Kobayashi K, et al. Hepatic glomerulonephritis. Characteristics of hepatic IgA glomerulonephritis as the major part. Virchows Arch A Pathol Anat Histol. 1981. 392(1): 45-54.
- Chan H, Tang Y, Lv X, et al. Risk Factors Associated with Renal Involvement in Childhood Henoch-Schönlein Purpura: A Meta-Analysis. PloS one. 2016. 11(11): e0167346-e0167346.
- Tan J, Tang Y, Xu Y, et al. The Clinicopathological Characteristics of Henoch-Schonlein Purpura Nephritis with Presentation of Nephrotic Syndrome. Kidney Blood Press Res. 2019. 44(4): 754-764.
- Oni L and Sampath S. Childhood IgA Vasculitis (Henoch Schonlein Purpura)-Advances and Knowledge Gaps. Frontiers in pediatrics. 2019. 7: 257.
- Yi F, Bai Z, Li Y, et al. A good response to glucocorticoid for Henoch-Schönlein purpura with abdominal pain and gastrointestinal bleeding in an adult: A CARE case report. Medicine (Baltimore). 2020. 99(1): e18602.
- Ogawa M, Makino Y, Ueda S, et al. Rapidly progressive glomerulonephritis in association with Henoch-Schonlein purpura in a patient with advanced liver cirrhosis. Nephron. 1995. 71(3): 365-6.
- Madison D, Allen E, Deodhar A, et al. Henoch-Schonlein purpura: a possible complication of hepatitis C related liver cirrhosis. Ann Rheum Dis. 2002. 61(3): 281-2.
- Akizue N, Suzuki E, Yokoyama M, et al. Henoch-Schonlein Purpura Complicated by Hepatocellular Carcinoma. Intern Med. 2017. 56(22): 3041-3045.
- Aggarwal M, Manske CL, Lynch PJ, et al. Henoch-Schonlein vasculitis as a manifestation of IgA-associated disease in cirrhosis. Am J Kidney Dis. 1992. 20(4): 400-214.15.
- Fleischmann C, Herrmann A, Felber J, et al. Multiple Hemorrhagic Skin Lesions in a 52-Year-Old Patient with Liver Cirrhosis. Dtsch Med Wochenschr. 2017. 142(17): 1273-1275.
- Miret Mas C, Gregori F, Segura R, et al. Shonlein-Henoch purpura as presenting form of alcoholic cirrhosis. Rev Clin Esp. 2003. 203(4): 213-5.
- Jung JH. Henoch-Schonlein purpura nephritis and colitis in an adult patient with alcoholic liver cirrhosis. Kidney Res Clin Pract. 2016. 35(3): 190-1.
- Gweon TG, Kwon, JH. Rapid progression and recovery of massive gastric mucosal damage complicated by Henoch-Schonlein purpura in a patient with liver cirrhosis: A case report. Exp Ther Med. 2019. 18(3): 2051-2054.
- Gatselis NK, Stefos A, Gioti C, et al. Primary biliary cirrhosis and Henoch-Schonlein purpura: report of two cases and review of the literature. Liver Int. 2007. 27(2): 280-3.
- Mu K, Zhang J, Gu Y, et al. Cord-derived mesenchymal stem cells therapy for liver cirrhosis in children with refractory Henoch-Schonlein purpura: A case report. Medicine (Baltimore). 2018. 97(47): e13287.
- Kitamura T, Nakase H, and Iizuka H. Henoch-Schonlein purpura after postoperative Staphylococcus aureus infection with hepatic IgA nephropathy. J Nephrol. 2006. 19(5): 687-90.
